- Open access
- Published: 16 July 2022

Physical activity during pregnancy: a systematic review for the assessment of current evidence with future recommendations
- Leona Cilar Budler 1 &
- Marko Budler 2 , 3
BMC Sports Science, Medicine and Rehabilitation volume 14 , Article number: 133 ( 2022 ) Cite this article
23k Accesses
18 Citations
54 Altmetric
Metrics details
Physical activity is essential to maternal and infant health. Healthcare professionals should inform pregnant women about benefits of physical activity to prevent possible health issues. Those recommendations should elaborate on relevant contemporary evidence. The aim of this study was to review evidence-based recommendations for physical activity during pregnancy.
A systematic search, analysis and synthesis of conducted randomised controlled trials (RCTs) was conducted from October 2021 to June 2022 in following databases: PubMed, CINAHL, ScienceDirect and Web of Science. Literature was searched using inclusion and exclusion criteria and following PRISMA recommendations.
Benefits for pregnant-women health and well-being were reported while performing aerobic exercise, lumbar stabilization and stretching exercise, water exercise, nerve and tendon-slip exercise, resistance training and strength training. For all exercise modalities it is recommended to perform moderate intensity activities during the whole time of pregnancy.
Conclusions
This systematic literature review supplements current knowledge on physical activity of pregnant women. Exercise interventions are listed and suggested in an integrative model with physical-fitness components to contextualize and promote physical activity among pregnant women.
Peer Review reports
Physical activity (PA) is defined as “bodily movement produced by skeletal muscles that results in energy expenditure” [ 1 ]. PA is believed to be essential to healthy pregnancy. Historically, Biblical writers noticed that Hebrew slave women gave birth more easily than sedentary Egyptian mistresses [ 2 ]. Moreover, it is believed that PA during pregnancy limits gestational weight-gain [ 3 , 4 , 5 ], decreases risk of maternal mental disorders after childbirth [ 6 , 7 ] and improves body image satisfaction [ 8 ]. PA in pregnancy is pivotal to facilitating positive health outcomes in infants [ 9 ].
To corroborate the role of PA in pregnancy and the expected favourable health outcomes for pregnant women and infants, this study distinguishes between the terms PA, exercise (intervention), and physical fitness that are all distinct concepts. However, these concepts (terms) are often used interchangeably. In line with a seminal paper, we deem exercise as “a subset of PA that is planned, structured, and repetitive and has as a final or an intermediate objective the improvement or maintenance of physical fitness”. In addition, physical fitness is deemed “a set of attributes that are either health- or skill-related” [ 10 ].
Pregnant women tend to demonstrate a lack of knowledge regarding PA during pregnancy [ 9 , 11 , 12 ]. The reasons for insufficient knowledge include but are not limited to the mothers’ race [ 13 ], socio-economic and cultural context [ 14 ], and maternal education [ 15 ]. Relatively low degree of pregnant women report that they had received prescription in terms of exercise interventions from health providers during pregnancy [ 16 ]. In addition, past research identified an important barrier to enhancing pregnant women’ knowledge about PA—the absence of PA-related domains in the development of professional healthcare professionals [ 17 ]. Due to lack of guidance, pregnant women access information about healthy lifestyle during pregnancy via the internet with questionable credibility [ 18 , 19 ].
Mottola et al. who performed a literature review and identified some benefits of prenatal PA, provided preliminary guidance for pregnant women and healthcare professionals on prenatal PAity [ 20 ]. Unsurprisingly, the women who were given guidelines for PA during pregnancy reported exercising [ 21 ].
In addition to potential issues with credible sources of information and lack of novel guidance, healthcare professionals need multiple sources to develop an “integrative approach” for promotion of PA to pregnant women [ 22 , 23 ]. From the standpoint of the authors,an “integrative approach” should, for instance, account for tailored exercise interventions that could be useful to pregnant women with specific goald, and facilitate the adherence of pregnant women to PA [ 24 , 25 ].
The aim of the current study is hence two-fold. First, we respond to more-recent calls for evidence-based recommendations that would enhance promotion of PA during pregnancy (see e.g., [ 26 ]). We have listed and contextualized the recommendations to be provided to pregnant women, professional healthcare professionals involved in nursing (e.g., midwives), and wider society. Further, by identifying exercise modalities, examining characteristics of exercise interventions with a systematic review, and corresponding health outcomes we address a recent call from DiPietro et al. [ 27 ] to advance current knowledge and empower healthcare professionals. As interventions should be tailored (e.g., goal-oriented to a physical-fitness components), our second aim is to characterize the interventions from main findings, and further elaborate characteristics such as exercise modality. By corroborating the exercise interventions for pregnant women, we can enhance the adherence to PA and improve the promotion of PA among the healthcare professionals. Ultimately, the current study concludes with the contributions to the learning system and an identification of intricate contemporary challenges to be addressed in the future research.
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize a specific health issue [ 28 ]. Following steps were taken into account when performing a systematic review: (1) defining research question; (2) preliminary literature search; (3) development of search string, inclusion and exclusion criteria; (4) literature search and analysis; (5) literature synthesis; (6) assessment of literature quality and bias; and (7) interpretation of findings and proposition of future directions. When performing a systematic review, the Additional file 1 : PRISMA guidelines were followed [ 25 ] (Additional file 1 : Appendix 1).
Search methods
For the development of research question, a Population, Intervention and Outcome—PICO [ 29 ] format was used. The research question was: How do exercise interventions improve the health of physically-active pregnant women and their infants? Exclusion and inclusion criteria were developed based on the preliminary literature search and PICO research question (Table 1 ).
We searched for literature published between June 2017 and June 2022, in English language and RCTs. As described by Hiebl [ 30 ], authors can limit their literature search by publication time. However, reasons should be justified and mentioned in the limitation section. The aim of this systematic review is to get the newest and best evidence in this field, thus, only literature published in last five years were examined. We provide up-to-date evidence which is often neglected by authors of overviews [ 31 ]. We included only RCTs because they are assessed as the most credible in the hierarchy of evidence [ 23 ]. Other studies were excluded (Additional file 1 ).
A search string was developed based on preliminary literature search and was taken into account in the process of literature search (Table 2 ).
Search outcomes
Using the developed search string 2092 records were identified in four databases. After duplicates exclusion, literature was checked by title and abstract; 26 articles were retrieved and checked by the full text. Finally, 20 articles were included in the final analysis and synthesis. Steps of literature search are presented in Fig. 1 [ 32 ].
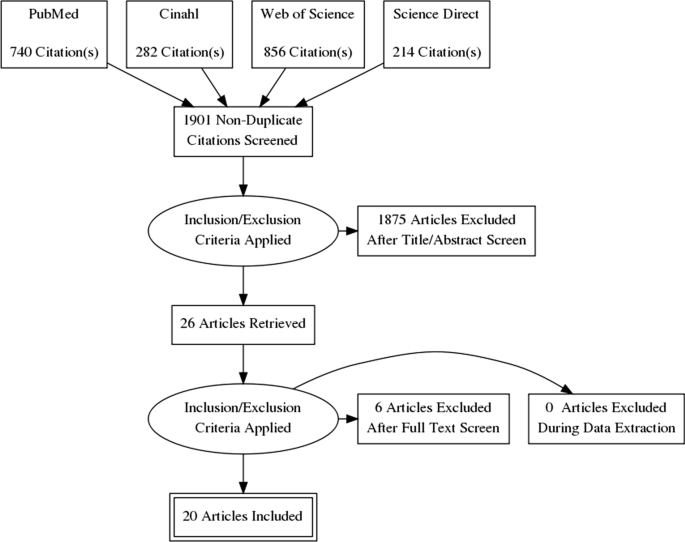
PRISMA flow diagram
Quality appraisal
The data quality was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system [ 33 , 34 ]. GRADE identified its five categories—study limitations, imprecision, inconsistency, indirectness, and publication bias. GRADE quality level is interpreted as high (++++), moderate (+++), low (++), or very low (+).
Data extraction
Data were extracted using standardized data form in Microsoft Excel® by two reviewers. The first reviewer exported data and second reviewer checked for data accuracy. The literature screening was carried out independently by two researchers. Disagreements were solved by consensus. We extracted study characteristics such as study sample, exercise intervention, main study results and conclusions.
Data synthesis
A narrative synthesis was conducted for all included studies. Results were synthesized by PA modality and intensity or duration of PA. Also, results were synthesised due to the intervention effectiveness.
In total, 20 articles were included in the final analysis (Table 3 ). Studies which fit the inclusion criteria were analysed due to sample, intervention, results and conclusions. Excluded studies are listed in the Additional file 2 : Appendix 2.
Studies included in the analyses involved various number of participants. Those numbers varied between minimum of 20 [ 27 ] and maximum of 1023 pregnant women [ 38 ]. All studies had intervention and control group. However, all studies did not report mean and standard deviation for each group. Interventions were the following: moderate aerobic exercise, lumbar stabilization and stretching exercise, resistance training, water exercise, various moderate-intensity exercises, physical conditioning program, cycling program, nerve and tendon-slip exercise, and individual or group sessions with a personal trainer. Studies outcomes are assessed as positive or negative for pregnant women or infant in the Table 4 . Details about each intervention are provided in the Table 5 . In the remainder of this study the observed studies are assessed for quality using the GRADE system (table in Additional file 3 : Appendix 3). All studies included were RCTs because of the search criteria. RCTs are seen as a high-quality body-of-knowledge [ 34 ]. When assessing quality of each evidence, study design, study limitations, inconsistency, indirectness, imprecision and risk of bias were considered. The results of the assessment are as follows. Among 18 units-of-analysis, three were assessed as moderate quality, eight as low quality, and seven as very low quality. In these seven, quality scored was lower due to numerous limitations (e.g., the absence of the control group, small study sample, lack of blinding, lack of robust analyses, etc.), higher deviations in CIs for interventions, or risk of bias.
Out of 20 identified RCTs, 11 (55.00%) reported positive results of implemented intervention on maternal or infant health outcomes (Table 4 ). Others showed no changes or did not report the result.
To help professional healthcare professionals in promoting PA we further categorize interventions by exercise modality (Table 5 ). Among a range of exercise modalities, four exercise modalities, namely strengthening, stretching, balance, and aerobic exercises, are commonly found in the existing body of literature that focuses on positive results of physical activity interventions on health outcomes [ 52 , 53 , 54 , 55 ].
Table 5 categorizes main findings with respect to exercise modality and their expected positive results on pregnant-women health and well-being. The favourable health outcomes of performing moderate aerobic exercise were most extensively examined [ 35 , 45 ], followed by strengthening see e.g., [ 42 ], or a hybrid form using both [ 38 , 44 ]. For aerobic exercise, treadmill, walking, and other aerobic-exercise programs are advised (see Table 5 ). A more comprehensive hybrid form of PA program included moderate aerobic exercise with gradual warm-up; aerobics; light muscle strengthening; balance; stretching; strengthening; and relaxation with final talk [ 35 ]. In addition, [ 56 ] proposed an exercise program of both aerobic and strength training among Norwegian pregnant women to examine vitamin-D mediated effect on maternal and fetal health outcomes.
Some of the remaining studies also focused on specific sub-types of, for instance, stretching [ 36 ], and aerobic exercise [ 47 ]. In fact, practitioners are advised to suggest pregnant women the tendon slip exercises and the nerve-gliding exercises. Gestational diabetes mellitus was the main research subject by [ 4 ]. Authors revealed that, complementary to healthy eating, exercise limits the gestational weight gain. What is more, their findings pinpoint to a comprehensive approach, comprised of, for instance, moderate PA, reducing sedentary time, and strengthening.
Fontana Carvalho et al. [ 36 ] reported that involved pregnant women were included in either lumbal stabilization exercise group or lower limb and trunk stretching exercise group. Both interventions showed positive results in pain reduction caused by or perceived as a result of pregnancy. Rodríguez-Blanque et al. [ 38 ] reported that pregnant women performed a moderate PA consisting of a warm-up; aerobic exercise, strengthening, and stretching with relaxation to limit the negative effects on the body and to optimise well-being, mood and sleep patterns. Finally, positive results were also seen by combing individual diet and PA [ 39 ]. Moreover, PA intervention entailed positive results in maternal diet quality. Similarly, [ 40 ] proposed a supervised PA program consisting of warm-up, aerobic exercise, strengthening, balance, and stretching with relaxation. Hereby, intensity of PA was mild-to-moderate. The PA showed positive results in weight loss at 6 weeks post-partum.
Among the combined exercise modalities, [ 35 ] introduced a 10-min warm-up (walking and stretching) with a main section that lasted 30–35 min and included moderate intensity aerobic and resistance exercises. Activity ended with a cool down (walking, stretching, relaxation and pelvic floor muscle training). Gustafsson et al. [ 45 ] proposed a 12-week standardized PA program where women were encouraged to perform exercise modalities at home at least twice a week. Fontana Carvalho et al. [ 36 ] reported success of exercise interventions with a combination of stabilization of lower limbs and stretching. With an aim of reducing carpal-tunnel symptoms, pregnant women also conducted tailored nerve and tendon slip exercises on daily basis [ 41 ]. With respect to duration, [ 44 ] suggested water exercise to be followed for 12 weeks. Finally, a PA program aimed at conditioning was developed to encourage pregnant women to perform exercises throughout the entire pregnancy [ 40 ].
Our findings reveal that past research on PA in pregnancy focused mostly on health outcomes for the pregnant women and infants. Similar to Evenson et al. (2014), our analysis revealed heterogeneity of findings in terms of exercise modality, duration, and intensity. To overcome some of these shortcomings, we call for a standardization in terms of measurable and comparative characteristics of PA in pregnancy. Following this train-of-thought, a commonly used FITT framework (see e.g., [ 57 ]) has been applied to numerous sub-domains where exercise prescriptions from the healthcare professionals play a significant role, ranging from patients with cancer [ 58 ], exercise prescriptions for cardiometabolic health [ 59 ], and occasionally as a one-size-fits-all to general population [ 60 ].
Applying a framework similar to FITT to pregnant women would allow for quantifying the common characteristics of PA in pregnancy (e.g., intensity). The development of such framework that would guide the hands-on recommendations from the healthcare professionals would require acknowledging for any challenges pregnant women might have as a result of, for instance, deteriorating health, lack of physical fit, and maturity of pregnancy [ 61 , 62 ]. Finally, a growing number of pregnant women have a propensity to remain physically fit notwithstanding pregnancy. Physical fitness is defined as a»state characterized by: (a) an ability to perform daily activities with vigour; and (b) demonstration of traits and capacities that are associated with low risk of premature development of physical inactivity [ 63 ]. In a broader sense, one’s physical fitness represents their capability to carry out a range of exercise modalities and daily tasks. Campbell et al. [ 64 ] emphasize the need to improve this capability concomitantly with the management any fatigue, stress, or change in health condition which is especially relevant assertion to the pregnant women. Moreover, there is a need for development of efficient physical activity interventions for pregnant women. Marini et al. [ 65 ] proposed a study protocol to design physical activity intervention for pregnant women to include in childbirth preparation classes evaluating its feasibility and efficacy on quality of life, PA levels and other outcomes.
The physical fitness is achieved, maintained and facilitated by prescribing exercise that accounts for the components of physical fitness. These components might differ with respect to the existing literature; however, most commonly the components are cardiorespiratory fitness [ 66 ], muscular strength [ 67 ], muscular endurance [ 68 ], body composition [ 69 ], and flexibility [ 55 , 70 ]. Elaborating on the physical-fitness components could reveal complementary outcomes (in addition to health outcomes identified by the current study and past research) relevant to pregnant women that aim to advance their PA. We suggest the future research to focus on further examination of the complementary role of maintaining physical fitness, to account for the associated health- and physical-fitness-related outcomes, and to overcome aforementioned context-based challenges for pregnant women (e.g., timing of PA).
The existing learning system in nursing can advance by moving towards an integrative approach that accounts for multiple sources, network collaboration, and continuous investigation of PA. We facilitate the learning processes of healthcare professionals by elaborating on exercise modalities, and commencing a discussion over the components of physical fitness. Acknowledging physical-fitness components could enhance adherence of pregnant women to PA (see e.g., [ 39 ]), and empower healthcare professionals who promote PA.
It is evident that research does not focus on different components of PA or components of physical fitness. In line with DiPietro et al. [ 27 ] we argue that the future research should focus on providing recommendations tailored for the »peri-pregnancy« period, i.e. before, during, and after a childbirth. Second; whilst the current study adds to tailoring exercise interventions by elaborating exercise modality and physical-fitness components, lack of detailed information not only about pregnant women but also about the pre-pregnancy condition prevents from optimizing interventions for particular cohorts of pregnant women.
Future research should focus on multiple sources to overcome aforementioned challenges that limit the development of tailored evidence-based PA recommendations. Furthermore, we suggest that, in addition to health outcomes, the future research devotes more attention to the role and plausibility of PA for achieving or maintaining physical-fitness components of pregnant women. Such enriched guidelines could enhance adherence and favourable health outcomes of pregnant women, and improve promotion of PA among the healthcare professionals. However, future research should identify appropriate healthcare professionals to distribute PA recommendations (see e.g., [ 23 ]), and, in addition, barriers that prevent from effective distribution of physical activity recommendations. Finally, to complement the existing body of the literature with PA recommendations for pregnant women, future research should ensure the scientific nature of such reviews by e.g., addressing the listed limitations, and expanding the data obtained.
The current study reveals a lack of context-dependency, for instance characteristics of pregnant women such as age, previous PA levels, comorbidities, other measures (e.g., BMI), mental well-being, pregnancy status (e.g., early or late pregnancy, health issues during pregnancy, micronutrient levels etc.), non-communicable diseases with viral infections (see e.g., [ 71 , 72 ]), and other factors such as personal attitude (see e.g., [ 73 ]) that could compromise the development and realization of PA. However, the current study also has some methodological limitations in addition to the limited feasibility of data analysis. First, we deliberately examined the more-recent literature in order to get novel and credible selection of evidence. We omitted analysing non-published papers or papers without a free access. Second, a heterogeneity of findings partially prevented from more thorough analyses. Finally, we did not use data processing software, which to some degree reduces the reliability of the qualitative analysis.
Conclusion with research agenda
The current study demonstrates numerous favourable health outcomes of PA during pregnancy. Recommendations given by practitioners to pregnant women focus on preforming at least 150 min per week moderate-intensity aerobic PA. However, further explanations are not provided. That being said, practitioners can use our systematic literature review to examine favourable maternal and infant health outcomes with a range of exercise modalities (strengthening, balance, stretching, and exercise modalities combined) in addition to aerobic exercise. Furthermore, the practitioners can learn from the current study about the importance of physical-fitness components. As adherence is consistently deemed a critical success factor for PA [ 74 ], accounting for the physical-fitness components, i.e., goal-oriented (tailored), in exercise interventions, could remarkably improve the adherence of pregnant women to PA.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Body mass index
Gestational diabetes mellitus
The grading of recommendations, assessment, development, and evaluation system
Number of participants
Oral glucose tolerance test
p value of statistical significance
Physical activity
Population, intervention and outcome
Randomised controlled trial
Study of water exercise during pregnancy
World Health Organization. What does “physical activity” mean? Geneva: WHO; 2022.
Google Scholar
Burnett CWF. Value of prenatal exercises. J Obstet Gynaecol. 1956;40:40–57.
Simmons D, et al. Effect of physical activity and/or healthy eating on GDM risk: the DALI lifestyle study. J Clin Endocrinol Metab. 2017;3:903–13.
Broekhuizen K, et al. Cost-effectiveness of healthy eating and/or physical activity promotion in pregnant women at increased risk of gestational diabetes mellitus: economic evaluation alongside the DALI study, a European multicenter randomized controlled trial. Int J Behav Nutr Phys Act. 2018;1:23.
Article Google Scholar
Mizgier M, et al. The impact of physical activity during pregnancy on maternal weight and obstetric outcomes. Ginekol Pol. 2018;89:80–8.
Article PubMed Google Scholar
Kołomańska D, Zarawski M, Mazur-Bialy A. Physical activity and depressive disorders in pregnant women—a systematic review. Medicina. 2019;5:212.
Shakeel N, et al. Physical activity in pregnancy and postpartum depressive symptoms in a multiethnic cohort. J Affect Disord. 2018;236:93–100.
Sun W, et al. Physical activity and body image dissatisfaction among pregnant women: a systematic review and meta-analysis of cohort studies. Eur J Obstet Gynecol Reprod Biol. 2018;229:38–44.
Santo EC, et al. Determinants of physical activity frequency and provider advice during pregnancy. BMC Pregnancy Childbirth. 2017;17:286.
Article PubMed PubMed Central Google Scholar
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31.
CAS PubMed PubMed Central Google Scholar
Grenier LN, et al. Be healthy in pregnancy: exploring factors that impact pregnant women’s nutrition and exercise behaviours. Matern Child Nutr. 2020;17:e13068.
PubMed PubMed Central Google Scholar
Whitaker KM, et al. African American and White women׳s perceptions of weight gain, physical activity, and nutrition during pregnancy. Midwifey. 2016;34:211–20.
da Silva GS, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;1:175.
O’Brien OA, et al. Influences on the food choices and physical activity behaviours of overweight and obese pregnant women: a qualitative study. Midwifery. 2017;47:28–35.
Akbari A, et al. Assessing of physical activity self-efficacy and knowledge about benefits and safety during pregnancy among women. RJMS. 2016;22:76–87.
Rodrigues Domingues M, Barros AJD. Leisure-time physical activity during pregnancy in the 2004 Pelotas Birth Cohort Study. Rev Saúde Pública. 2007;41:173–80.
Hopkinson Y, et al. Midwives understanding of physical activity guidelines during pregnancy. Midwifery. 2018;59:23–6.
Cannon S, et al. A review of pregnancy information on nutrition, physical activity and sleep websites. Women Birth. 2020;33:35–40.
Murray DM, Fisher JD. The internet: a virtually untapped tool for research. J Technol Hum Serv. 2022;19:5–18.
Mottola MF, Davenport MH, Ruchat SM, Davies GA, Poitras VJ, Gray CE, Jaramillo Garcia A, Barrowman N, Adamo KB, Duggan M, Barakat R, Chilibeck P, Fleming K, Forte M, Korolnek J, Nagpal T, Slater LG, Stirling D, Zehr L. 2019 Canadian guideline for physical activity throughout pregnancy. Br J Sports Med. 2018;52:1339–46.
May LE, et al. Exercise during pregnancy: the role of obstetric providers. J Osteopath Med. 2013;113:612–9.
Hamilton K, et al. Being active in pregnancy: theory-based factors associated with physical activity among pregnant women. Women Health. 2019;2:213–28.
De Vivo M, Mills H. “They turn to you first for everything”: insights into midwives’ perspectives of providing physical activity advice and guidance to pregnant women. BMC Pregnancy Childbirth. 2019;1:1–2.
Evenson KR, Mottola MF, Artal R. Review of recent physical activity guidelines during pregnancy to facilitate advice by health care providers. Obstet Gynecol Surv. 2019;8:481–9.
Lindqvist M, Persson M, Mogren I. “Longing for individual recognition”–pregnant women’s experiences of midwives’ counselling on physical activity during pregnancy. Sex Reprod Healthc. 2018;1:46–53.
Lindqvist M, Persson M, Mogren I. “Longing for individual recognition”–pregnant women’s experiences of midwives’ counselling on physical activity during pregnancy. Sex Reprod Healthc. 2018;15:46–53.
DiPietro L, Evenson KR, Bloodgood B, Sprow K, Troiano RP, Piercy KL, Vaux-Bjerke A, Powell KE. Benefits of physical activity during pregnancy and postpartum: an umbrella review. Med Sci Sports Exerc. 2019;51:1292.
Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;1:9–14.
Schardt C, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:1–6.
Hiebl MRW. Sample selection in systematic literature reviews of management research. Organ Res Methods. 2021. https://doi.org/10.1177/1094428120986851 .
Pieper D, Antoine SL, Neugebauer EA, Eikermann M. Up-to-dateness of reviews is often neglected in overviews: a systematic review. J Clin Epidemiol. 2014;67:1302–8.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Andrews JC, et al. GRADE guidelines: 15. Going from evidence to recommendation—determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66:726–35.
Balshem H, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6.
Barakat R, et al. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz J Phys Ther. 2019;23:148–55.
Fontana Carvalho AP, et al. Effects of lumbar stabilization and muscular stretching on pain, disabilities, postural control and muscle activation in pregnant woman with low back pain. Eur J Phys Rehabil Med. 2020;3:297–306.
de Vargas Nunes Coll C, et al. Efficacy of regular exercise during pregnancy on the prevention of postpartum depression: the PAMELA randomized clinical trial. JAMA Netw Open. 2019;1:e186861.
Rodríguez-Blanque R, et al. Water exercise and quality of life in pregnancy: a randomised clinical trial. Int J Environ Res Public Health. 2020;4:1288.
Dodd JM, Deussen AE, Louise J. A randomised trial to optimise gestational weight gain and improve maternal and infant health outcomes through antenatal dietary, lifestyle and exercise advice: the OPTIMISE randomised trial. Nutrients. 2019;12:2911.
Brik M, et al. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet Gynecol. 2019;5:583–9.
Keskin Y, et al. Effectiveness of home exercise in pregnant women with carpal tunnel syndrome: randomized control trial. J Pak Med Assoc. 2020;2:202–7.
OʼConnor PJ, et al. Effects of resistance training on fatigue-related domains of quality of life and mood during pregnancy: a randomized trial in pregnant women with increased risk of back pain. Psychosom Med. 2018;3:327–32.
Krohn Garnæs K, et al. Effects of supervised exercise training during pregnancy on psychological well-being among overweight and obese women: secondary analyses of the ETIP-trial, a randomised controlled trial. BMJ Open. 2019;11:e028252.
Backhausen MG, et al. The effects of an unsupervised water exercise program on low back pain and sick leave among healthy pregnant women: a randomised controlled trial. PLoS ONE. 2017;9:e0182114.
Article CAS Google Scholar
Gustafsson MK, et al. The effect of an exercise program in pregnancy on vitamin D status among healthy, pregnant Norwegian women: a randomized controlled trial. BMC Pregnancy Childbirth. 2019;1:1–10.
Atkinson SA, et al. Be Healthy in Pregnancy (BHIP): a randomized controlled trial of nutrition and exercise intervention from early pregnancy to achieve recommended gestational weight gain. Nutrients. 2022;14:810.
de Andrade Leão OA, Domingues MR, Bertoldi AD, Ricardo LIC, de Andrade Müller W, Tornquist L, Martins RC, Murray J, Silveira MF, Crochemore-Silva I, Hallal PC, Mielke GI. Effects of regular exercise during pregnancy on early childhood neurodevelopment: the physical activity for mothers enrolled in longitudinal analysis randomized controlled trial. J Phys Act Health. 2022;19:203–10.
Gonzalez-Plaza E, Bellart J, Arranz Á, Luján-Barroso L, Mirasol EC, Seguranyes G. Effectiveness of a step counter smartband and midwife counseling intervention on gestational weight gain and physical activity in pregnant women with obesity (Pas and Pes study): randomized controlled trial. JMIR Mhealth Uhealth. 2022;10:e28886.
Talebi E, Mohaddesi H, Vahabzadeh D, et al. Examination of influence of social media education through mobile phones on the change in physical activity and sedentary behavior in pregnant women: a randomized controlled trial. BMC Womens Health. 2022;22:152.
Sandborg J. HealthyMoms : a smartphone application to promote healthy weight gain, diet and physical activity during pregnancy : a randomized controlled trial. Huddinge: s.l.: Karolinska Institutet; 2022.
Niaraki MR, Pakniat H, Alizadeh A, Hosseini MA, Ranjkesh F. Effect of exercise in water on the musculoskeletal pain in pregnant women: a randomized controlled trial. J Musculoskelet Res. 2021;24:2150003.
Hayden JA, et al. Some types of exercise are more effective than others in people with chronic low back pain: a network meta-analysis. J Physiother. 2021;67:252–62.
Shimada IS, et al. Effects of exercise therapy on painful temporomandibular disorders. J Oral Rehabil. 2019;46:475–81.
Murphy SE, et al. Influence of exercise type on maternal blood pressure adaptation throughout pregnancy. AJOG Glob Rep. 2022;2:100023.
Haskell WL, Montoye HJ, Orenstein D. Physical activity and exercise to achieve health-related physical fitness components. Public Health Rep. 1985;100:202.
Embaby H, Elsayed E, Fawzy M. Insulin sensitivity and plasma glucose response to aerobic exercise in pregnant women at risk for gestational diabetes mellitus. Ethiop J Health Sci. 2016;5:409–14.
Flynn AC, Seed PT, Patel N, Barr S, Bell R, Briley AL, Godfrey KM, Nelson SM, Oteng-Ntim E, Robinson SM, Sanders TA, Sattar N, Wardle J, Poston L, Goff LM, UPBEAT consortium. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int J Behav Nutr Phys Act. 2016;13:124.
Kirkham AA, et al. Exercise prescription and adherence for breast cancer: one size does not FITT all. Med Sci Sports Exerc. 2018;50:177–86.
Reid RER, Thivel D, Mathieu M-E. Understanding the potential contribution of a third “T” to FITT exercise prescription: the case of timing in exercise for obesity and cardiometabolic management in children. Appl Physiol Nutr Metab. 2019;44:911–4.
Burnet K, et al. How fitting is FIIT? A perspective on a transition from the sole use if frequency, intensity, time, and type in exercise prescription. Psychol Behav. 2019;199:33–4.
CAS Google Scholar
Malta MB, et al. Educational intervention regarding diet and physical activity for pregnant women: changes in knowledge and practices among health professionals. BMC Pregnancy Childbirth. 2016;16:1–9.
De Vivo M, Mills H. Laying the foundation for pregnancy physical activity profiling: a framework for providing tailored physical activity advice and guidance to pregnant women. Int J Environ Res Public Health. 2021;18:5996.
Pate RR. The evolving definition of physical fitness. Quest. 1988;40:147–79.
Campbell N, De Jesus S, Prapavessis H. Physical fitness. In: Gellman ME, Turner JR, editors. Encyclopedia of behavioral medicine. New York: Springer; 2013. p. 95–136.
Marini S, et al. Co-design and evaluation of the feasibility and the efficacy of a multiple-targeted adapted physical activity intervention to promote quality of life, well-being and physical activity levels in pregnant women: the “WELL-DONE!” study protocol. Sustainability. 2021;13:12285.
Marín-Jiménez N, et al. Association of self-reported physical fitness with pain during pregnancy: the GESTAFIT project. Scand J Med Sci Sports. 2019;29:1022–30.
PubMed Google Scholar
O’Connor PJ, et al. Safety and efficacy of supervised strength training adopted in pregnancy. J Phys Act Health. 2011;8:309–20.
White E, Pivarnik J, Pfeiffer K. Resistance training during pregnancy and perinatal outcomes. J Phys Act Health. 2014;11:1141–8.
Henriksson P, et al. Associations of body composition and physical fitness with gestational diabetes and cardiovascular health in pregnancy: results from the HealthyMoms trial. Nutr Diabetes. 2021;11:16.
Article CAS PubMed PubMed Central Google Scholar
Figueira HA, et al. Pregnancy-related low back pain relief after maximum static flexibility program. Health. 2014;6:2966–72.
Zovko V, Budler M. Sitting ducks: physical activity and diet-related interventions in the" peri-COVID-19" period. Kinesiol Slov. 2021;27:52–72.
Sitzberger C, et al. Physical activity in high-risk pregnancies. J Clin Med. 2022;3:703.
Room J, et al. What interventions are used to improve exercise adherence in older people and what behavioural techniques are they based on? A systematic review. BMJ Open. 2017;12:e019221.
Download references
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Faculty of Health Sciences, University of Maribor, Zitna ulica 15, 2000, Maribor, Slovenia
Leona Cilar Budler
School of Economics and Business, University of Ljubljana, Kardeljeva ploscad 17, 1000, Ljubljana, Slovenia
Marko Budler
Fitness Association of Slovenia, Cesta 24. junija 23, 1231, Ljubljana, Slovenia
You can also search for this author in PubMed Google Scholar
Contributions
LCB and MB were involved in the study conceptualization and design of the systematic review. LCB and MB were responsible for generating the systematic review terms, performing the systematic searches, extracting the data, analysing the data, performing the data synthesis, and for creating the study, tables and figures. Both authors have read and approved the final study.
Corresponding author
Correspondence to Leona Cilar Budler .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Appendix 1.
Additional file 2.
Appendix 2.
Additional file 3.
Appendix 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Cilar Budler, L., Budler, M. Physical activity during pregnancy: a systematic review for the assessment of current evidence with future recommendations. BMC Sports Sci Med Rehabil 14 , 133 (2022). https://doi.org/10.1186/s13102-022-00524-z
Download citation
Received : 27 March 2022
Accepted : 12 July 2022
Published : 16 July 2022
DOI : https://doi.org/10.1186/s13102-022-00524-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Intervention
- Health outcome
BMC Sports Science, Medicine and Rehabilitation
ISSN: 2052-1847
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial ☆ ☆☆
Ruben barakat, ignacio refoyo, javier coteron, evelia franco.
- Author information
- Article notes
- Copyright and License information
Corresponding author at: Martín Fierro 7, 28040 Madrid, Spain. [email protected]
Received 2018 May 9; Accepted 2018 Nov 6; Issue date 2019 Mar-Apr.
Exercise in pregnancy is associated with better control of maternal weight gain.
Moderate regular exercise throughout pregnancy prevents gestational diabetes.
Healthy pregnant women should be encourage to exercise regularly.
Keywords: Exercise, Physical therapy, Pregnancy, Weight gain, Gestational diabetes
Excessive gestational weight gain is associated with several adverse events and pathologies during pregnancy.
The purpose of this study was to examine the effects of an exercise program throughout pregnancy on maternal weight gain and prevalence of gestational diabetes.
A randomized controlled trial was designed that included an exercise intervention group (EG) and standard care control group (CG). The exercise intervention included moderate aerobic exercise performed three days per week (50–55 minutes per session) for 8–10 weeks to 38–39 weeks gestation.
594 pregnant women were assessed for eligibility and 456 were included (EG n = 234; CG n = 222). The results showed a higher percentage of pregnant women gained excessive weight in the CG than in the EG (30.2% vs 20.5% respectively; odds ratio, 0.597; 95% confidence interval, 0.389–0.916; p = 0.018). Similarly, the prevalence of gestational diabetes was significantly higher in the CG than the EG (6.8% vs 2.6% respectively; odds ratio, 0.363; 95% confidence interval, 0.138–0.953; p = 0.033).
The results of this trial indicate that exercise throughout pregnancy can reduce the risk of excessive maternal weight gain and gestational diabetes.
Introduction
Pregnancy and delivery are biological processes that can have a significant impact on maternal health and newborn wellbeing. Research has shown that events that occur during pregnancy may influence both maternal and fetal future health outcomes. 1 , 2
The impact that gestational weight gain can have on health outcomes has been especially recognized by health care professionals as a potential factor that may influence maternal and fetal wellbeing. Excessive gestational weight gain is associated with several adverse events and pathologies. Many studies report complications related to the wellbeing of the mother, fetus and even the newborn and infant due to inappropriate maternal weight gain during pregnancy. 3 , 4 , 5 , 6 , 7 , 8
Gestational diabetes mellitus (GDM) is defined as “carbohydrate intolerance with onset or first recognition during pregnancy” 9 and it is among many problems that are highly related to excessive maternal weight gain. 10 Indeed the prevalence of GDM is increasing in parallel with overweight and obesity in the obstetric population. 11 , 12 Current trends for weight gain among women of reproductive age are alarming. 13 , 14
Precise estimates of GDM prevalence are not clear. A recent meta-analysis reported that the prevalence of GDM in Europe is 5.4%. 15 According to the American Diabetes Association (ADA), GDM complicates approximately 7% of all pregnancies. 16 Regardless of the variability presented in available studies, data from western countries suggests that the prevalence of GDM is increasing. 17 , 18 , 19 Women diagnosed with GDM have a higher risk for future diabetes, with approximately 50% of women developing type 2 diabetes within 5 years of delivery. 20
Many studies support the association of GDM with several adverse maternal and fetal outcomes. 21 , 22 , 23 Additionally, there are some data that suggest an increase in fetal malformation and perinatal mortality. 24 , 25 , 26
Although research supports that healthy lifestyle modifications may have a positive impact on metabolic factors among overweight and obese pregnant women, evidence for specific effective approaches to prevent GDM are needed. 27 Research to identify modifiable factors that might help prevent excessive maternal weight gain and abnormal glucose tolerance or GDM, in the pregnant population is needed and has urgent public health importance. 28 , 29 One such modifiable factor may be exercise performed during pregnancy.
The existing literature suggests that physical activity before and during pregnancy may be an effective public health and clinical strategy for GDM prevention and treatment. 30 This effect might be explained by the widely accepted influence that physical activity has on preventing weight gain. 31
Research has supported exercise during pregnancy as an effective intervention to prevent excessive gestational weight gain. 32 Furthermore, exercise during pregnancy has been identified as an effective approach to control blood sugars to help prevent and manage GDM. 33 Previous studies carried out with pregnant women however have conducted physical activity programs using small sample sizes and/or lacking supervision. 34 , 35
The main aim of this randomized controlled trial (RCT) was to examine the influence of a supervised exercise program throughout pregnancy on maternal weight gain and incidence of GDM. As a secondary objective, the effect of the exercise program on other maternal and neonatal outcomes was also examined. We hypothesized that maternal physical exercise would be associated with a reduction of both excessive maternal weight gain and prevalence of GDM without adverse effects on other maternal and newborn outcomes.
The present RCT (clinical trial registration number NCT02109588 ) was conducted between March 2014 and January 2017 following the ethical guidelines of the Declaration of Helsinki, last modified in 2000. The research protocol was reviewed and approved by the Hospital Severo Ochoa (Madrid, Spain) ethics review board (240-09). Participants enrollment began in April 2014.
Participants and randomization
A total of 594 Spanish-speaking (Caucasian) healthy pregnant women from two primary care medical centers ( Centro de Salud Los Pedroches, Centro de Salud Leganés Norte , Madrid, Spain) were recruited during their first prenatal visit ( Fig. 1 ). They were informed about the nature of the study and assessed for eligibility. Women with singleton and uncomplicated pregnancies (no type 1, 2 or gestational diabetes at baseline), with no history or risk of preterm delivery (i.e. ≥1 previous preterm delivery) and not participating in any other trial were invited to participate. Women not planning to give birth in the same obstetric hospital, or with no medical follow-up throughout pregnancy were not included in the study. Women having any serious medical conditions (contraindications) that prevented them from exercising safely were also not included. 36
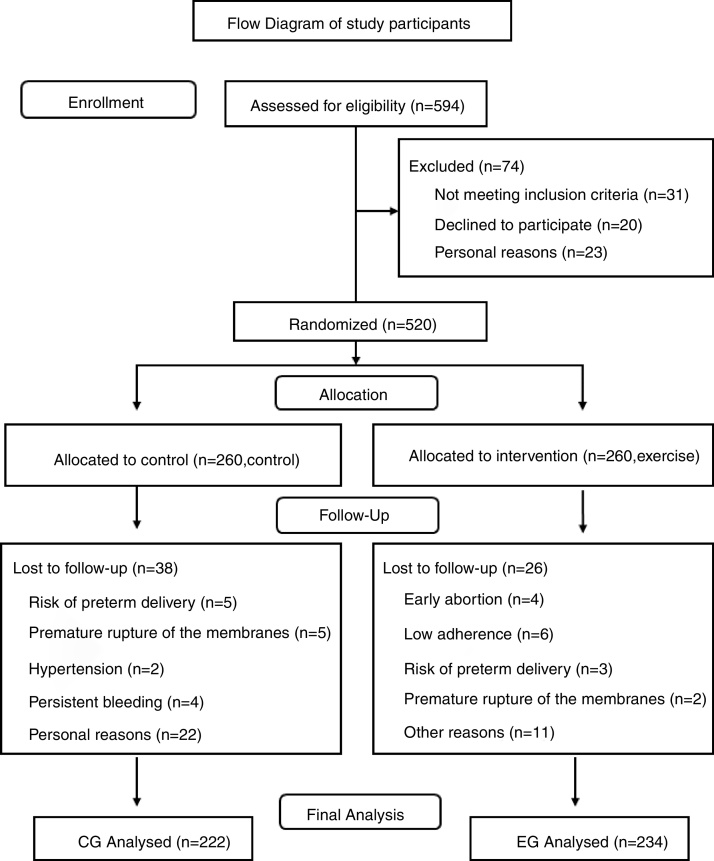
Flow chart of study participants.
A computer-generated list of random numbers was used to allocate the participants into the study groups following other previous studies. Allocation ratio was 1:1. The randomization blinding process (sequence generation, allocation concealment and implementation) was performed by three different researchers. The treatment allocation system was set up so that the researcher who was in charge of randomly assigning participants to each group did not know in advance which treatment the next person would receive (i.e. concealed allocation).
Women who were randomly allocated to the Exercise Group (EG) received similar standard care and performed an exercise program throughout pregnancy. Women randomly allocated to the Control Group (CG) received obstetric standard care from health professionals. Women were excluded if they did not conform to the specifications of the allotted group. All the participants signed an informed consent.
Exercise intervention 37 , 38
Pregnant women in the intervention group received standard care and all aspects of a structured and supervised moderate exercise intervention program three days per week (55–60 min per session) from the 8–10th week of pregnancy (immediately after the first prenatal ultrasound) to the end of the third trimester (weeks 38–39). The exercise protocol was supervised by a qualified of physical activity and sport science professional (ten years of experience). A total of 83–85 group training sessions were originally planned for each participant in the event of no preterm delivery. The exercise program met the standards of the American College of Obstetricians and Gynecologists 36 and included the following seven sections:
Gradual warm-up
Aerobic exercises
Light muscle strengthening
Coordination and balance exercises
Stretching exercises
Pelvic floor strengthening
Relaxation and final talk
Women used a heart rate (HR) monitor (Accurex Plus, Finland) during the training sessions (HR was consistently under 70% of age-predicted maximum) and the rating of perceived exertion scale ranged from 12 to 14 (Somewhat Hard). 39
The exercise session started with a light-intensity, 10-min warm-up consisting of walking and static stretching (avoiding muscle pain) of most muscle groups (upper and lower limbs, neck and trunk muscles). Similarly, the exercise session finished with a light-intensity, 10-min cool-down including the same exercises as the warm-up period plus relaxation and pelvic floor muscle training. As a motivational strategy, a final talk was done to promote extensive counseling and provide information to ensure that the participants received clear instructions on how to have an active pregnancy and emphasizing the importance of regular (not occasional) exercise throughout pregnancy.
The main section of the exercise session after the warm-up was 30–35 min in length and included moderate-intensity aerobic exercises and resistance exercises. Aerobic exercises consisted of low-impact aerobic dance, involving the upper and lower limbs. Aerobic dance bouts were approximately 3–4 min long and included stretching and relaxation followed by a one minute break.
Light muscle strengthening was also included in each session. Strengthening exercises engaged major muscle groups (pectoral, back, shoulder, upper and lower limb muscles) to promote good posture, prevent low back pain and strengthen the muscles used in labor and the pelvic floor (third trimester). Exercises were performed using the full range of motion and involved barbells (3 kg/exercise) and low-medium-resistance elastic bands (Therabands). The exercises included biceps curls, arm extensions, arm side lifts, shoulder elevations, bench presses, seated lateral row, lateral leg elevations, leg circles, knee extensions, knee (hamstring) curls, ankle flexions and extensions. Exercises involving extreme stretching and joint over-extension, ballistic movements or jumps were avoided, and exercises in the supine position on the floor were not performed for more than 2 min.
As pregnancy progresses, women may experience difficulty with balance therefore all coordination and balance exercises consisted of easy activities using sport equipment (foam balls, cords, etc.) for support.
To maximize program safety, adherence and efficacy, all sessions were: (i) supervised by a qualified fitness specialist (ten years of experience) and with an obstetrician's assistance; (ii) accompanied by music; and (iii) performed in the Health Care Center in a spacious, well-lit room under favorable environmental conditions (altitude 600 m; temperature 19–21 °C; humidity 50–60%). An adequate intake of calories and nutrients was confirmed before the start of each exercise session.
The intervention involved group sessions of 12–15 participants.
Adherence to the training program was ≥80% in the intervention group that was measured by a qualified fitness specialist using a checklist of attendance for each session.
Standard-care (CG)
The women assigned to the standard care CG attended regular scheduled visits to their obstetricians and midwives (according to Hospital protocol), usually every 4–5 weeks until the 36–38th week of gestation and then weekly until delivery. They received general nutrition and physical activity counseling from their health-care provider.
Women were not discouraged from exercising during pregnancy on their own. However, similar to our previous studies women in the CG were asked about exercise habits once each trimester using a “Decision Algorithm” (by telephone). 37
Participant demographics
Information about demographics, including pre-pregnancy Body Mass Index (BMI), parity, educational level, previous physical activity habits, smoking status, previous pre-term birth and previous miscarriage was obtained at the first prenatal visit either by reviewing the medical records or by a telephone interview. The inclusion/exclusion criteria was determined at this initial visit by the attending obstetrician.
Primary outcomes
Total maternal weight gain (kg) and excessive gestational weight gain (yes/no) were recorded. Total gestational weight gain was calculated on the basis of the pregravid weight (first prenatal consult) and weight at the last clinic visit before delivery (week 36–38). Excessive gestational weight gain was defined according to the recommendations of the 2009 Institute of Medicine (IOM) guidelines 40 categorized by pre-pregnancy BMI for each woman: >18 kg for underweight; >16 kg for normal weight; >11.5 kg for overweight; and >9 kg for obese women. Cases of gestational diabetes and 1 h Oral Glucose Tolerance Test (OGTT) information was collected from hospital records (week 24–26).

Secondary outcomes
Maternal gestational age at delivery, type of delivery and birth weight were collected from hospital records. Newborns were classified as having macrosomia when birth weight was >4000 × g and low birth weight was defined as <2500 × g . 41 Primary and secondary outcomes were assessed by healthcare professionals.
Statistical analyses
Sample size was determined based on a priori widely accepted power calculation. 42 In total, 340 subjects were needed to achieve 80% power to detect a statistically significant difference in maternal weight gain taking into account previous data on this variable. The sample size was intentionally increased to account for patient withdrawal and possible problems for follow-up.
A Kolmogorov–Smirnov test was performed to verify the normality of the data in the study variables and showed that it was non-parametric ( p < 0.05). Thus, Mann–Whitney tests were performed to analyze possible differences between the groups for continuous variables (maternal weight gain, oral glucose tolerance test (OGTT), maternal age, gestational age, pre-pregnancy BMI and birthweight). The Pearson χ 2 test was completed with the observation of standardized adjusted residuals and was used to assess differences between categorical variables (excessive weight gain, gestational diabetes, parity, mode of delivery). Statistical tests used a 2-sided 0.05 alpha level and SPSS 24.0 was used to analyze the data. All analyses were done on an intention-to-treat basis.
Results ( Fig. 1 )
Baseline characteristics.
Baseline characteristics for both groups are listed in Table 1 and were similar between groups for most of the variables.
Maternal characteristics.
Main outcomes
Differences in main outcomes (maternal weight gain, OGTT and cases of GDM) are presented in Table 2 . Maternal weight gain was significantly lower in the EG compared to the CG (12.19 vs 13.33 kg respectively, U = 22044, p = 0.005). In line with these results, standardized adjusted residuals in Pearson χ 2 suggested that the ratio of women that gained excessively was higher in the CG than the EG (30.2% vs 20.5% respectively; odds ratio, 0.597; 95% confidence interval, 0.389–0.916; p = 0.018). A significant difference was also found for the OGTT results (EG = 116.56 vs CG = 121.63 mg/dL, U = 23,158, p = 0.045). Finally, standardized adjusted residuals in Pearson χ 2 suggested that the ratio of women diagnosed with GDM was higher in the CG than the EG (6.8% vs 2.6% respectively; odds ratio, 0.363; 95% confidence interval, 0.138–0.953; p = 0.033).
Maternal weight gain, oral glucose tolerance test and gestational diabetes.
Kilograms (kg).
OGTT: oral glucose tolerance test.
Milligrams per deciliter (mg/dL).
Other maternal and neonatal outcomes
Other outcomes of interest analyzed in the study are presented in Table 3 . Among maternal outcomes, no differences were found for gestational age, number of preterm deliveries or mode of delivery. In regards to newborn outcomes, no differences were found for birthweight between study groups. Our results showed that, although the χ 2 test was not significant, the ratio of neonate macrosomia was slightly higher in the CG than in the EG (7.2% vs 3.4% respectively; odds ratio, 0.456; 95% confidence interval, 0.191–1.087).
Other maternal and newborn outcomes.
The aim of the present study was to examine whether regular and supervised physical exercise during pregnancy can influence prevention of excessive maternal weight gain, and GDM, which are both closely related factors. Similar to our previous work, the main strength of the current study is the combination of light resistance, toning, aerobic dance, coordination, stretching and pelvic floor muscle training in the same program throughout pregnancy and examining the resultant effects on outcomes. The main finding of this study is that the exercise program reduced the total (mean) maternal weight gain as well as the cases of excessive weight gain and GDM.
Our results are relevant from a clinical and health care point of view due to the increasing prevalence of these two parameters in recent years, in parallel with the alarming rise of worldwide overweight and obesity. 11 , 12 Furthermore the interpretation of our results promote the use of moderate and supervised physical exercise throughout pregnancy as a method to increase prevention of pregnancy complications and improve quality of life for pregnant women without adverse effects on maternal and fetal well-being.
Regarding the external validity and generalizability of our findings the high adherence (≥80% attendance) of this large RCT for all pre-pregnancy BMI categories strongly supports the extension of the present results to the healthy pregnant population.
In regards to the newborn health outcomes, although birth weight was similar in neonates between the CG and the EG, the percentage of newborns with macrosomia was lower in the EG. We had previously observed 37 , 38 this effect, and therefore this study provides additional evidence that physical exercise may improve perinatal outcomes by preventing excessive accumulation of weight during fetal development.
Other authors have previously investigated the impact of prenatal exercise on excessive gestational weight gain and GDM. 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 Among the great variety of study designs used, RCTs are the most reliable as they allow management of independent variables (exercise program design). Current literature available on RCTs includes a great variety of exercise programs used. It might explain the difficulty in determining the exact type and frequency of exercise during pregnancy that is required to prevent and treat GDM.
From a methodological point of view the more adaptive/desirable outcomes are reported by those studies in which a supervised intervention (exercise program) including a large variety of exercises (aerobic, resistance, pelvic floor and muscle strengthening, stretching, etc.) have been provided throughout the pregnancy. 46 , 47 , 48 , 49 , 50 , 51
Regardless of the variability among exercise interventions, most researchers agree that prenatal exercise is an excellent way for controlling maternal weight gain during pregnancy. Our results are in consensus with many authors, 43 , 44 , 45 , 46 and with our previous studies on this health outcome. 47 , 48
However, as we mentioned previously the relationship between exercise and GDM has been unclear. While some evidence suggests a high efficacy in the use of exercise as a preventive method, 49 , 50 , 51 literature has been inconsistent on the effect of prenatal exercise when used as a treatment method for reducing risk factors for GDM. 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 Differences in exercise programs may explain this. In our opinion the variance in the duration of the programs, length of the sessions, adherence and especially the type of exercises used, contribute to the differences observed in the results of studies.
Strengths and limitations
The major strengths of our study include the large number of participants in this RCT, the high adherence to intervention (>80% attendance) and the identification of those women in the CG who did not remain sedentary. In our opinion, the present results provide healthcare practitioners with evidence-based information that can be used to recommend supervised physical exercise throughout pregnancy to maintain or improve the quality of life of pregnant women including labor and birth.
One limitation of the current study was that nutrition or energy intake was not assessed, however, all pregnant women had (by their obstetricians and midwives) standard care which included regular information about a healthy lifestyle during pregnancy including nutrition information. Therefore the supervised exercise program was the only difference between study groups. In addition, we found differences between the study groups for parity and educational level of participants which could potentially influence the results.
The impracticality of instituting this type of a supervised activity program for pregnant women on a mass scale may be another potential limitation of the present study. Furthermore, our study focused on a Spanish population and was conducted in two tertiary care hospitals in Madrid, which may lower the external validity of our findings.
We conclude that a supervised physical exercise program initiated early and maintained throughout pregnancy can reduce the risk of excessive maternal weight gain and GDM.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the technical assistance of the Gynecology and Obstetrics Department of “Hospital Severo Ochoa” and the health practitioners of Centro de Salud Los Pedroches, Centro de Salud Leganés Norte, Madrid, Spain.
The authors also would like to acknowledge the technical assistance for the English revision to Taniya Singh Nagpal from University of Western Ontario (Canada).
This paper is part of a Special Issue on Women's Health Physical Therapy.
Trial Identifier: NCT02109588 . https://clinicaltrials.gov/ct2/show/NCT02109588
- 1. Artal R., O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37:6–12. doi: 10.1136/bjsm.37.1.6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Gillman M.W., Rifas-Shiman S., Berkey S., Field A.E., Colditz G.A. Maternal gestational diabetes, birthweigt, and adolescent obesity. Pediatrics. 2003;111(3):221–226. doi: 10.1542/peds.111.3.e221. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Fraser A., Tilling K., Macdonald-Wallis C. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Ali Z., Nilas L., Ulrik C.S. Excessive gestational weight gain in first trimester is a risk factor for exacerbation of asthma during pregnancy: a prospective study of 1283 pregnancies. J Allergy Clin Immunol. 2018 Feb;141(2):761–767. doi: 10.1016/j.jaci.2017.03.040. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Liu K., Ye K., Han Y. Maternal and cord blood fatty acid patterns with excessive gestational weight gain and neonatal macrosomia. Asia Pac J Clin Nutr. 2017;26(2):291–297. doi: 10.6133/apjcn.012016.11. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Jharap V.V., Santos S., Steegers E.A.P., Jaddoe V.W.V., Gaillard R. Associations of maternal obesity and excessive weight gain during pregnancy with subcutaneous fat mass in infancy. Early Hum Dev. 2017 May;108:23–28. doi: 10.1016/j.earlhumdev.2017.03.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Subhan F.B., Colman I., McCargar L., Bell R.C., APrON Study Team Higher pre-pregnancy BMI and excessive gestational weight gain are risk factors for rapid weight gain in infants. Matern Child Health J. 2017;21(6):1396–1407. doi: 10.1007/s10995-016-2246-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Blackwell S.C., Landon M.B., Mele L. Relationship between excessive gestational weight gain and neonatal adiposity in women with mild gestational diabetes mellitus. Obstet Gynecol. 2016;128(6):1325–1332. doi: 10.1097/AOG.0000000000001773. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl. 1):S62–S69. doi: 10.2337/dc11-S062. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG. 2010;117:575–578. doi: 10.1111/j.1471-0528.2009.02486.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Chu S.Y., Callaghan W.M., Kim S.Y. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–2207. doi: 10.2337/dc06-2559a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Nery C., Moraes S.R.A., Novaes K.A., Bezerra M.A., Silveira P.V.C., Lemos A. Effectiveness of resistance exercise compared to aerobic exercise without insulin therapy in patients with type 2 diabetes mellitus: a meta-analysis. Braz J Phys Ther. 2017;21(6):400–415. doi: 10.1016/j.bjpt.2017.06.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Kaaja R., Rönnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. Rev Diabet Stud. 2008;5(4):194–202. doi: 10.1900/RDS.2008.5.194. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Chen A., Xu F., Xie C. Gestational weight gain trend and population attributable risks of adverse fetal growth outcomes in Ohio. Paediatr Perinat Epidemiol. 2015;29(4):346–350. doi: 10.1111/ppe.12197. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Eades C.E., Cameron D.M., Evans J.M.M. Prevalence of gestational diabetes mellitus in Europe: a meta-analysis. Diabetes Res Clin Pract. 2017;129:173–181. doi: 10.1016/j.diabres.2017.03.030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. American Diabetes Association Standards of medical care in diabetes – 2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Luoto R.M., Kinnunen T.I., Aittasalo M. Prevention of gestational diabetes: design of a cluster-randomized controlled trial and one-year follow-up. BMC Pregnancy Childbirth. 2010;10:39. doi: 10.1186/1471-2393-10-39. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Dabelea D., Snell-Bergeon J.K., Hartsfield C.L., Bischoff K.J., Hamman R.F., McDuffie R.S. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28(3):579–584. doi: 10.2337/diacare.28.3.579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Kjos S.L. Postpartum care of the woman with diabetes. Clin Obstet Gynecol. 2000;43:75–86. doi: 10.1097/00003081-200003000-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Ogle C. Gestational diabetes: real risks beyond the controversy. Midwifery Today Int Midwife. 2013;(108):57–58. [ PubMed ] [ Google Scholar ]
- 22. Jang H.C., Cho H.C., Min Y.K. Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in Korean women with GDM. Diabetes Care. 1997;20:1582–1588. doi: 10.2337/diacare.20.10.1582. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Hapo Study Cooperative Research Group Hyperglycaemia and adverse pregnancy outcomes. N Eng J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Sepe S.J., Connell F.A., Geiss L.S. Gestational diabetes: incidence, maternal characteristics, and perinatal outcome. Diabetes. 1985;34(Suppl. 2):13–16. doi: 10.2337/diab.34.2.s13. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Schaefer U., Songster G., Xiang A. Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy. Am J Obstet Gynecol. 1997;177:1165–1171. doi: 10.1016/s0002-9378(97)70035-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Schmidt M.I., Spichler E.R., Duncan B.B. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001;24:1151–1155. doi: 10.2337/diacare.24.7.1151. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Callaway L.K., Colditz P.B., Byrne N.M. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33(7):1457–1459. doi: 10.2337/dc09-2336. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Oken E., Ning Y., Rifas-Shiman S.L., Radesky J.S., Rich-Edwards J.W., Gillman M.W. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol. 2006;108(5):1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Chasan-Taber L., Marcus B.H., Stanek E., 3rd A randomized controlled trial of prenatal physical activity to prevent gestational diabetes: design and methods. J Womens Health (Larchmt) 2009;18(6):851–859. doi: 10.1089/jwh.2008.1006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Ramos-Leví A.M., Pérez-Ferre N., Fernández M.D. Risk factors for gestational diabetes mellitus in a large population of women living in Spain: implications for preventative strategies. Int J Endocrinol. 2012:312529. doi: 10.1155/2012/312529. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Lee I., Djoussé L., Sesso H.D., Wang L., Buring J.E. Physical activity and weight gain prevention. JAMA. 2010;303(12):1173–1179. doi: 10.1001/jama.2010.312. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Wiebe H.W., Boulé N.G., Chari R., Davenport M.H. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet Gynecol. 2015 May;125(5):1185–1194. doi: 10.1097/AOG.0000000000000801. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Mottola MF1, Artal R. Role of exercise in reducing gestational diabetes mellitus. Clin Obstet Gynecol. 2016;59(3):620–628. doi: 10.1097/GRF.0000000000000211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Simmons D., Devlieger R., van Assche A. Effect of physical activity and/or healthy eating on GDM risk: the DALI lifestyle study. J Clin Endocrinol Metab. 2017;102(3):903–913. doi: 10.1210/jc.2016-3455. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Daly N., Farren M., McKeating A., OʼKelly R., Stapleton M., Turner M.J. A medically supervised pregnancy exercise intervention in obese women: a randomized controlled trial. Obstet Gynecol. 2017;130(5):1001–1010. doi: 10.1097/AOG.0000000000002267. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. ACOG Committee Opinion No. 650: physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126(6):e135–142. doi: 10.1097/AOG.0000000000001214. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Barakat R., Pelaez M., Cordero Y. Exercise during pregnancy protects against hypertension and macrosomia. Randomized Clinical Trial. Am J Obstet Gynecol. 2016;214(5):649. doi: 10.1016/j.ajog.2015.11.039. e1–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Barakat R., Franco E., Perales M., López C., Mottola M.F. Exercise during pregnancy is associated with a shorter duration of labor. A randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:33–40. doi: 10.1016/j.ejogrb.2018.03.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. O’Neill M.E., Cooper K.A., Mills C.M., Boyce E.S., Hunyor S.N. Accuracy of Borg's ratings of perceived exertion in the prediction of heart rates during pregnancy. Br J Sports Med. 1992;26(2):121–124. doi: 10.1136/bjsm.26.2.121. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Institute of Medicine . National Academies Press; Washington, DC: 2009. Weight Gain during Pregnancy: Reexamining the Guidelines. 324p. [ PubMed ] [ Google Scholar ]
- 41. Stetzer B.P., Thomas A., Amini S.B., Catalano P.M. Neonatal anthropometric measurements to predict birth weight by ultrasound. J Perinatol. 2002;22(5):397–402. doi: 10.1038/sj.jp.7210754. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Röhrig B., du Prel J.B., Watchlin D., Kwiecien R., Blettner M. Sample size calculation in clinical trials. Dtsch Arztebl Int. 2010;107(31–32):552–556. doi: 10.3238/arztebl.2010.0552. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Nobles C., Marcus B.H., Stanek E.J. The effect of an exercise intervention on gestational weight gain: the behaviors affecting baby and you (B.A.B.Y) study. A randomized controlled trial. Am J Health Prom. 2018;32:736–744. doi: 10.1177/0890117117732409. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Da Silva S., Curi P., Rodriguez M. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;14(1):175. doi: 10.1186/s12966-017-0632-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Sagedal L.R., Øverby N.C., Bere E. Lifestyle intervention to limit gestational weight gain: the Norwegian Fit for Delivery randomized controlled trial. BJOG. 2017;124:97–109. doi: 10.1111/1471-0528.13862. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Herring S.J., Cruice J.F., Bennett G.G., Rose M.Z., Davey A., Foster G.D. Preventing excessive gestational weight gain among African American women: a randomized clinical trial. Obesity (Silver Spring) 2016;24:30–36. doi: 10.1002/oby.21240. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Bacchi M., Mottola M., Perales M., Refoyo I., Barakat R. Aquatic activities during pregnancy prevent excessive maternal weight gain and preserve birth weight: a randomized clinical trial. Am J Health Promot. 2017;32:729–735. doi: 10.1177/0890117117697520. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Ruiz J.R., Perales M., Pelaez M., Lopez C., Lucia A., Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc. 2013;88:1388–1397. doi: 10.1016/j.mayocp.2013.07.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Wang C., Wei Y., Zhang X. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;4:340–351. doi: 10.1016/j.ajog.2017.05.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Garnæs K.K., Mørkved S., Salvesen Ø., Moholdt T. Exercise training and weight gain in obese pregnant women: a randomized controlled trial (ETIP Trial) PLoS Med. 2016;13:e1002079. doi: 10.1371/journal.pmed.1002079. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Barakat R., Pelaez M., Lopez C., Lucia A., Ruiz J.R. Exercise during pregnancy and gestational diabetes-related adverse effects: a randomized controlled trial. Br J Sports Med. 2013;47:630–636. doi: 10.1136/bjsports-2012-091788. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Hawkins M., Hosker M., Marcus B.H. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: a feasibility randomized controlled trial. Diabet Med. 2015;32:108–115. doi: 10.1111/dme.12601. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Oostdam N., van Poppel M.N.M., Wouters M.G.A.J. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomized controlled trial. BJOG. 2012;119:1098–1107. doi: 10.1111/j.1471-0528.2012.03366.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Korpi-Hyövälti E.A.L., Laaksonen D.E., Schwab U.S. Feasibility of lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health. 2011;11:179–187. doi: 10.1186/1471-2458-11-179. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Luoto R., Kinnunen T.I., Aittasalo M. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLoS Med. 2011;8:e1001036. doi: 10.1371/journal.pmed.1001036. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (824.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Exercise in Pregnancy
A Clinical Review
Sally K Hinman , MD, PhD
Kristy b smith , md, david m quillen , md, m seth smith , md, pharmd.
- Author information
- Article notes
- Copyright and License information
Sally K. Hinman, MD, PhD, Department of Community Health and Family Medicine, University of Florida, 1707 North Main Street, Gainesville, FL 32609 (email: [email protected] ).
Issue date 2015 Nov.
Health professionals who care for pregnant women should discuss potential health benefits and harms of exercise. Although most pregnant women do not meet minimal exercise recommendations, there are a growing number of physically active women who wish to continue training throughout pregnancy.
Evidence Acquisition:
A search of the Web of Science database of articles and reviews available in English through 2014. The search terms exercise pregnancy, strenuous exercise pregnancy , and vigorous exercise pregnancy were used.
Study Design:
Clinical review.
Level of Evidence:
With proper attention to risk stratification and surveillance, exercise is safe for the mother and fetus. Benefits of exercise in pregnancy include reduction in Cesarean section rates, appropriate maternal and fetal weight gain, and managing gestational diabetes. Exercise as a means of preventing gestational diabetes, preeclampsia, or perinatal depression cannot be reliably supported. Overall, the current evidence suffers from a lack of rigorous study design and compliance with physical activity interventions.
Conclusion:
Research thus far has been unable to consistently demonstrate proposed benefits of exercise in pregnancy, such as preventing gestational diabetes, preeclampsia, or perinatal depression. However, moderate- and high-intensity exercise in normal pregnancies is safe for the developing fetus and clearly has several important benefits. Thus, exercise should be encouraged according to the woman’s preconception physical activity level.
Keywords: pregnancy, exercise, strenuous, review
Historically, pregnant women were considered vulnerable and were advised to reduce their level of activity. 28 In 2002, the American College of Obstetricians and Gynecologists (ACOG) updated their recommendations for exercise during pregnancy to be less restrictive 1 ; these recommendations were reaffirmed by the ACOG in 2009. However, a survey of physicians found that more than 60% of physicians were not familiar with the current ACOG guidelines for exercise during pregnancy. 9 Although limited by a small sample size that included physicians in both obstetrics and gynecology and family medicine in 1 geographic region, this study highlights the deficiency in knowledge regarding this subject. 9
In general, exercise reduces the morbidity and mortality associated with cardiovascular disease, hypertension, and type 2 diabetes mellitus among other chronic diseases. 39 Nevertheless, only 20.3% of American adults meet weekly exercise recommendations. 40 Similarly, compliance with physical activity guidelines is low both prior to and during pregnancy. 3 Additionally, studies have consistently shown that women tend to decrease their physical activity during pregnancy. 3 , 24 , 30 Since pregnancy itself is a life-changing event for many women, it is also a time when other lifestyle changes may be enacted, such as smoking cessation, adopting a healthy diet, or beginning routine exercise. Additionally, as female participation in sports increases, 45 the safety of training during pregnancy has become an important issue.
Effects of Exercise on Maternal and Fetal Health in Pregnancy
Exercise offers potential benefits to both maternal and fetal health. 5 , 6 , 17 , 18 , 21 , 25 - 27 , 32 , 35 , 36 , 38 , 42 , 52 , 54
Gestational Diabetes Mellitus
In parallel to its effect on the incidence of type 2 diabetes mellitus, regular exercise should also decrease the risk of gestational diabetes mellitus. 21 However, several review articles have concluded that there is insufficient evidence to support physical activity as an effective intervention to decrease the risk of developing gestational diabetes. 29 , 48 , 53 Poor compliance to exercise regimens may have contributed to the lack of significance. 48 Nevertheless, multiple studies have shown significantly lower glucose levels on the 24- to 28-week oral glucose tolerance test in physically active women. 5 , 18 Although physical activity may not prevent the development of gestational diabetes, it may help manage it. The majority of studies using exercise as an intervention to treat gestational diabetes mellitus were successful. 48 Women diagnosed with gestational diabetes at 24 to 34 weeks of pregnancy who performed resistance exercise were less likely to require insulin during the remainder of their pregnancy as compared with women with gestational diabetes in the control group. 17 Additionally, exercise modulates maternal weight gain in pregnancy 27 and reduces the risk of large-for–gestational age newborns, 32 , 42 , 52 which are concerns with gestational diabetes.
Hypertension and Preeclampsia
Hypertension and preeclampsia are significant sources of morbidity and mortality in pregnancy. 46 Although physical activity is known to be helpful in preventing cardiovascular disease, a similar association between physical activity in pregnancy and hypertension or preeclampsia has not been definitively shown. Data reported from the North Carolina Pregnancy Risk Assessment Monitoring System indicate that gestational hypertensive complications are less likely in women who are physically active before and during pregnancy. 38 Conversely, an increased risk of developing preeclampsia was shown with greater than 270 minutes of exercise per week in a prospective cohort study of 85,139 pregnant Danish women. 41 A 2012 review of randomized control, cohort, and case-control studies suggests that there is a trend toward a preventive effect of physical activity on the development of preeclampsia. 36 However, there were a dearth of studies, and the evaluation of the few studies was complicated by differing methodologies, including the quantification of physical activity and the diagnosis of preeclampsia.
Maternal-Fetal Circulation and Fetal Growth
There is theoretical concern that exercise may negatively impact the developing fetus in terms of hemodynamics and growth. 7 , 20 , 27 However, this is unsubstantiated in the current literature. Multiple studies have shown that blood flow to the fetus is not significantly altered by moderate-intensity physical activity. 7 , 20 , 23 , 50 Interestingly, an increase in total vascular volume, capillary surface area, and parenchymal density was demonstrated in the placentas of women delivering at term who had exercised during the first half or all of their pregnancy. 31 Overall, birth weight was not significantly different between physically active women and inactive women. 20 , 32 , 50 , 52 Additionally, several studies have demonstrated that women who were physically active had a decreased risk of having babies that were large for gestational age. 32 , 42 , 52 Although additional studies would be beneficial, research thus far indicates that physical activity is safe for the developing fetus.
Labor and Delivery
Regular exercise may shorten the duration of labor and reduce the risk of Cesarean section and operative-assisted vaginal delivery. 6 , 35 Improved tone of abdominal and pelvic floor musculature and aerobic fitness may be important factors. Evidence-based support for this is limited, 6 , 35 as there are few contradictory results. 8 Women who participated in an exercise program throughout their pregnancies had a lower percentage of Cesarean section and instrumental vaginal deliveries compared with a control group. 6 This was in contrast to an earlier randomized controlled trial showing that there was no significant difference in Cesarean section and instrumental vaginal deliveries for women participating in an exercise program compared with a control group. 8 However, the exercise program was only from weeks 20 to 36 of gestation compared with weeks 6 to 39 in the later study. In another study, aerobic fitness was tested only in nulliparous women, which can affect labor duration, and a higher maximal oxygen consumption (VO 2 max) as a measure of aerobic fitness was associated with an approximately 30-minute shorter labor duration. 35
Perinatal Depression
Since exercise is associated with fewer depressive symptoms in adults with clinical depression, 37 it has also been hypothesized that exercise would alleviate symptoms of depression during pregnancy 19 , 25 , 26 , 47 and postpartum. 16 Although several studies report a decrease in depressive symptoms on questionnaires in women who are physically active, 19 , 25 , 26 , 47 the findings are not consistent. One study showed that pregnant women who were exercising 1 to 2 times per week, but not 3 times or more per week, were less likely to report depression, 26 while another study reported decreased depression in pregnant women who exercised 4 times or more per week but not less than 4 times per week. 25 Additionally, it is not clear whether the lower depression scores reported are clinically significant. 47 A meta-analysis of 5 randomized controlled trials concluded that there is insufficient evidence to determine whether exercise reduces symptoms of postpartum depression. 16
Guidelines for Weight Gain in Pregnancy
Weight gain is tracked throughout pregnancy as it has important ramifications on both maternal and fetal health. Excessive weight gain is associated with gestational diabetes, preeclampsia, and postpartum weight retention. 27 In 2013, the ACOG endorsed the Institute of Medicine’s weight gain goals during pregnancy based on a woman’s body mass index (BMI) at her first prenatal visit. 2 According to these recommendations, women with a normal BMI (18.5-24.9 kg/m 2 ) should gain 25 to 35 pounds whereas overweight (BMI 25-29.9 kg/m 2 ) and obese (BMI >30 kg/m 2 ) women should aim to gain 15 to 25 pounds and 11 to 20 pounds, respectively. 2 In 1 study, approximately 40% of normal-weight women and 60% of overweight women gained more weight than the upper limit of the respective range recommended by the Institute of Medicine. 14 Exercise can help manage weight gain during pregnancy. Women who attended all 24 supervised exercise sessions during a 12-week program stayed within the Institute of Medicine’s weight gain guidelines compared with 62% of the control group. 27 Overall, the compliant members of the exercise group had significantly less weight gain and postpartum weight retention compared with the control group. 27
Guidelines for Exercise in Pregnancy
The ACOG reaffirmed their 2002 committee opinion on exercise in pregnancy in 2009. 1 Based on these recommendations, women who are currently physically active can continue exercising while those who are physically inactive are encouraged to start exercising. 1 The American College of Sports Medicine’s (ACSM) physical activity guidelines calling for “30 minutes or more of moderate exercise . . . on most, if not all, days of the week” is advised for normal pregnancies. 1 Sixteen percent of pregnant women meet these recommendations; for comparison, only 26% of nonpregnant women comply with the ACSM physical activity recommendations. 44 Additionally, pregnant women should be cautioned to avoid exercise in the supine position after the first trimester and exercise involving prolonged standing due to significant decreases in cardiac output 15 ; exercise with a high risk of contact, falling, or abdominal trauma due to the risk of injury to the mother or the fetus 4 ; exercise at altitudes greater than 5250 feet due to concerns for fetal hypoxemia 22 ; and scuba diving due to the risk of the fetus developing decompression sickness. 1 , 12 Table 1 provides a summary of the exercise guidelines.
Summary of guidelines for exercise during pregnancy a
These guidelines apply to uncomplicated pregnancies. Please see text for the absolute and relative contraindications to exercise in pregnancy.
Exercise Prescription
Before advising the initiation or continuation of physical activity during pregnancy, a physician must assess the woman’s risk level. Healthy women without contraindications to exercise are considered low risk regardless of their previous activity level, whereas women with certain chronic medical conditions, including cardiovascular, respiratory, and systemic diseases, or relative contraindications are considered high risk. 10 , 13 Familiarity with absolute and relative contraindications to exercise is thus important for both the physician and patient. Absolute contraindications include gestational hypertension, preeclampsia, ruptured membranes, incompetent cervix, bleeding in the second or third trimester, multiple gestation at risk for premature labor, placenta previa, and premature labor. 1 Relative contraindications include intrauterine growth restriction, extremes of weight, and poorly controlled medical comorbidities, such as type 1 diabetes mellitus, hypertension, seizure disorder, and thyroid disease. 1 Pregnant women should also stop exercising based on signs and symptoms that may develop ( Table 1 ). 1 , 54
The frequency, intensity, type, and time duration (FITT) should be outlined according to her physical activity state prior to pregnancy. The PARmed-X for pregnancy is a set of guidelines developed in Canada to help health practitioners evaluate a pregnant woman’s ability to safely engage in physical activity as well as prescribe a basic exercise regimen. 43
Physical Training in Pregnancy
With more women competing in sports, strenuous exercise in pregnancy has become an important topic to those who wish to continue training during pregnancy. According to the ACOG, the main concerns in this population are the effect of pregnancy on competing and the effect of training on the pregnancy. 1 Continuing high-intensity exercise throughout pregnancy significantly increased the participants’ VO 2 max from week 17 gestation to 12 weeks postpartum 34 ; this training regimen of muscle strengthening, aerobic exercise, and endurance exercise could be used to guide exercise prescription for physically active women.
Although there are limited studies that examine the safety of high-intensity physical activity on the fetus, the results are mostly reassuring. 33 , 50 , 51 There was a decreased risk of preterm birth with increased frequency of first trimester vigorous recreational physical activity, and birth weight was not significantly affected by this level of physical activity. 33 Women classified as nonexercisers, moderately active, or vigorously active based on their physical activity 6 months prior to pregnancy performed a moderate-intensity exercise test during the second trimester. 50 As postexercise biophysical profiles were normal and there were no significant differences among the groups in APGAR scores or birth weights, this study suggests that physically inactive women can safely start moderate-intensity exercise while physically active women can continue or increase their activity to vigorous-intensity exercise during pregnancy. Additionally, the 3 groups of women performed a peak exercise test to detect the effect of strenuous physical activity on fetal well-being. 51 Again, biophysical profiles were ultimately reassuring in all groups. Notably, in 5 vigorously active women, there were transient fetal heart rate decelerations as well as altered uterine blood flow immediately after exercise, which raises concerns of reduced blood flow to the uterus with strenuous exercise. Similarly, in a small study of 6 pregnant Olympic-level endurance athletes, maternal-fetal circulation during and after exercise 49 showed that vigorous exercise, where the maternal heart rate was greater than 90% of the maximum, was associated with decreased uterine artery blood flow and fetal bradycardia that resolved soon after exercise was stopped. 11 , 51 Thus, even though there may be fetal cardiovascular changes associated with high-intensity exercise, they do not appear to significantly affect neonatal outcomes; however, there is cause for concern.
For women without contraindications to physical activity, exercise is safe for both the woman and developing fetus. Although there is no conclusive evidence that exercise effectively prevents gestational diabetes mellitus, preeclampsia, or perinatal depression, it does appear to have beneficial effects on reducing glucose levels, the risk of Cesarean section or instrumental vaginal deliveries, and maternal weight gain. In general, women who are physically active prior to pregnancy should be advised to maintain, and counseled that they can increase, their level of activity if desired, while physically inactive women should be encouraged to begin exercising.

The authors report no potential conflicts of interest in the development and publication of this article.
- 1. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the post-partum period. Obstet Gynecol. 2002;99:171-173. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013;121:210-212. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Amezcua-Prieto C, Olmedo-Requena R, Jimenez-Mejias E, et al. Changes in leisure time physical activity during pregnancy compared to the prior year. Matern Child Health J. 2013;17:632-638. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Artal R, Sherman C. Exercise during pregnancy: safe and beneficial for most. Phys Sportsmed. 1999;27:51-75. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomized controlled trial. Br J Sports Med. 2012;46:656-661. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Barakat R, Pelaez M, Lopez C, Montejo R, Coteron J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: results of a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:2372-2376. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Barakat R, Ruiz JR, Rodríguez-Romo G, Montejo-Rodríguez R, Lucia A. Does exercise training during pregnancy influence fetal cardiovascular responses to an exercise stimulus? Insights from a randomized, controlled trial. Br J Sports Med. 2010;44:762-764. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Barakat R, Ruiz JR, Stirling JR, Zakynthinaki M, Lucia A. Type of delivery is not affected by light resistance and toning exercise training during pregnancy: a randomized controlled trial. Am J Obstet Gynecol. 2009;201:590.e1-e6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Bauer PW, Broman CL, Pivarnik JM. Exercise and pregnancy knowledge among healthcare providers. J Womens Health (Larchmt). 2010;19:335-341. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Bredin SS, Foulds HJ, Burr JF, Charlesworth SA. Risk assessment for physical activity and exercise clearance: in pregnant women without contraindications. Can Fam Physician. 2013;59:515-517. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Bung P, Huch R, Huch A. Maternal and fetal heart rate patterns: a pregnant athlete during training and laboratory exercise tests; a case report. Eur J Obstet Gynecol Reprod Biol. 1991;39:59-62. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Camporesi EM. Diving and pregnancy. Semin Perinatol. 1996;20:292-302. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Charlesworth S, Foulds HJ, Burr JF, Bredin SS. Evidence-based risk assessment and recommendations for physical activity clearance: pregnancy. Appl Physiol Nutr Metab. 2011;36(suppl 1):S33-S48. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004-2005: fueling future obesity. Am J Obstet Gynecol. 2009;200:271.e1-e7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Clark SL, Cotton DB, Pivarnik JM, et al. Position change and central hemodynamic profile during normal third-trimester pregnancy and post partum. Am J Obstet Gynecol. 1991;164:883-887. Erratum in: Am J Obstet Gynecol . 1991;165:241. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Daley A, Jolly K, MacArthur C. The effectiveness of exercise in the management of post-natal depression: systematic review and meta-analysis. Fam Pract. 2009;26:154-162. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestation diabetes mellitus. Am J Obstet Gynecol. 2010;203:556.e1-e6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Deierlein AL, Siega-Riz AM, Evenson KR. Physical activity during pregnancy and the risk of hyperglycemia. J Womens Health (Larchmt). 2012;21:769-775. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Demissie Z, Siega-Riz AM, Evenson KR, Herring AH, Dole N, Gaynes BN. Physical activity and depressive symptoms among pregnant women: the PIN3 study. Arch Womens Ment Health. 2011;14:145-157. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. de Oliveria Melo AS, Silva JL, Tavares JS, Barros VO, Leite DF, Amorim MM. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol. 2012;120:301-310. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Dye TD, Knox KL, Artal R, Aubry RH, Wojtowycz MA. Physical activity, obesity, and diabetes in pregnancy. Am J Epidemiol. 1997;146:961-965. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Entin PL, Coffin L. Physiologic basis for recommendations regarding exercise during pregnancy at high altitude. High Alt Med Biol. 2004;5:321-334. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Ertan AK, Schanz S, Tanriverdi HA, Meyberg R, Schmidt W. Doppler examinations of fetal and uteroplacental blood flow in AGA and IUGR fetuses before and after maternal physical exercise with the bicycle ergometer. J Perinatal Med. 2004;32:260-265. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med. 2011;53:39-43. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Gaston A, Prapavessis H. Tired, moody and pregnant? Exercise may be the answer. Psychol Health. 2013;28:1353-1369. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Gjestland K, Bo K, Owe KM, Eberhard-Gran M. Do pregnant women follow exercise guidelines? Prevalence data among 3482 women, and prediction of low-back pain, pelvic girdle pain and depression. Br J Sports Med. 2013;47:515-520. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Haakstad LA, Bo K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC Pregnancy Childbirth. 2011;11:66. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Hammer RL, Perkins J, Parr R. Exercise during the childbearing year. J Perinatal Educ. 2000;9:1-14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7:CD009021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Hegaard HK, Damm P, Hedegaars M, et al. Sports and leisure time physical activity during pregnancy in nulliparous women. Matern Child Health J. 2011;15:806-813. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Jackson MR, Gott P, Lye SJ, Ritchie JW, Clapp JF., 3rd The effects of maternal aerobic exercise on human placental development: placental volumetric composition and surface areas. Placenta. 1995;16:179-191. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Juhl M, Olsen J, Andersen PK, Nohr EA, Andersen AM. Physical exercise during pregnancy and fetal growth measures: a study within the Danish National Birth Cohort. Am J Obstet Gynecol. 2010;202:63.e1-e8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Jukic AM, Evenson KR, Daniela JL, Herring AH, Wilcox AJ, Hartmann KE. A prospective study of the association between vigorous physical activity during pregnancy and length of gestation and birthweight. Matern Child Health J. 2012;16:1031-1044. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Kardel KR. Effects of intense training during and after pregnancy in top-level athletes. Scand J Med Sci Sports. 2005;15:79-86. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Kardel KR, Johanse B, Voldner N, Iversen PO, Henriksen T. Association between aerobic fitness in late pregnancy and duration of labor in nulliparous women. Acta Obstet Gynecol Scand. 2009;88:948-952. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Kasawara KT, do Nascimento SL, Costa ML, Surita FG, e Silva JL. Exercise and physical activity in the prevention of pre-eclampsia: systematic review. Acta Obstet Gynecol Scand. 2012;91:1147-1157. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Krogh J, Nordentoft M, Sterne JA, Lawlor DA. The effect of exercise in clinically depressed adults: systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2010;72:529-538. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Martin CL, Brunner Huber LR. Physical activity and hypertensive complications during pregnancy: findings from 2004 to 2006 North Carolina Pregnancy Risk Assessment Monitoring System. Birth. 2010;37:202-210. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Melzer K, Keyser B, Pichard C. Physical activity: the health benefits outweigh the risks. Curr Opin Clin Nutr Metab Care. 2004;7:641-647. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. National Center for Health Statistics. Health, United States, 2013: with special feature on prescription drugs. Hyattsville, MD. 2014. http://www.cdc.gov/nchs/data/hus/hus13.pdf#068 . Accessed January 18, 2015. [ PubMed ]
- 41. Østerdal ML, Strøm M, Klemmensen AK, et al. Does leisure time physical activity in early pregnancy protect against pre-eclampsia? Prospective cohort in Danish women. BJOG. 2009;116:98-107. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Owe KM, Nystad W, Bo K. Association between regular exercise and excessive newborn birth weight. Obstet Gynecol. 2009;114:770-776. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. PAR-Q+ Collaboration. The electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Vancouver, British Columbia, Canada. 2011. http://www.eparmedx.com . Accessed April 30, 2015.
- 44. Petersen AM, Leet TL, Brownson RC. Correlates of physical activity among pregnant women in the United States. Med Sci Sports Exerc. 2005;37:1748-1753. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Pivarnik JM, Perkins CD, Moyerbrailean T. Athletes and pregnancy. Clin Obstet Gynecol. 2003;46:403-414. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437-445. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Robledo-Colonia AF, Sandoval-Restrepo N, Mosquera-Valderrama YF, Escobar-Hurtado C, Ramírez-Vélez R. Aerobic exercise training during pregnancy reduces depressive symptoms in nulliparous women: a randomized trial. J Physiother. 2012;58:9-15. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Ruchat SM, Mottola MF. The important role of physical activity in the prevention and management of gestational diabetes mellitus. Diabetes Metab Res Rev. 2013;29:334-346. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Salvensen KA, Hem E, Sundgot-Borgen J. Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br J Sports Med. 2012;46:279-283. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Szymanski LM, Satin AJ. Exercise during pregnancy: fetal responses to current public health guidelines. Obstet Gynecol. 2012;119:603-610. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Szymanski LM, Satin AJ. Strenuous exercise during pregnancy: is there a limit? Am J Obstet Gynecol. 2012;207:179.e1-e6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Tomic V, Sporis G, Tomic J, Milanovic Z, Zigmundovac-Klaic, Pantelic S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat Med J. 2013;54:3620368. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Yin YN, Li XL, Tao TJ, Luo BR, Liao SJ. Physical activity during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Br J Sports Med. 2014;48:290-295. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Zarorsky GS, Longo LD. Exercise guidelines in pregnancy: new perspectives. Sports Med. 2011;41:345-360. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (679.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Physical exercise during pregnancy: a systematic review
Affiliation.
- 1 Obstetric Unit, Department of Obstetrics and Gynecology, University of Campinas, Campinas, Sao Paulo, Brazil. [email protected]
- PMID: 23014142
- DOI: 10.1097/GCO.0b013e328359f131
Purpose of review: This review aims to provide an update on the recent evidence concerning exercise during pregnancy including effects for mother and fetus and the types, frequency, intensity, duration and rate of progression of exercise performed.
Recent findings: Exercises during pregnancy are associated with higher cardiorespiratory fitness, prevention of urinary incontinence and low back pain, reduced symptoms of depression, gestational weight gain control, and for cases of gestational diabetes, reduced number of women who required insulin. There is no association with reduction in birth weight or preterm birth rate. The type of exercise shows no difference on results, and its intensity should be mild or moderate for previous sedentary women and moderate to high for active women. The exercise recommendations still are based on the current guidelines on moderate-intensity, low-impact, aerobic exercise at least three times a week. Yet, new guidelines propose increasing weekly physical-activity expenditure while incorporating vigorous exercise and adding light strength training to the exercise routine of healthy pregnant women. In the case of other chronic diseases like hypertension, there are still few data, and therefore more studies should be performed to assess the safety of the intervention.
Summary: Physical exercise is beneficial for women during pregnancy and also in the postpartum period; it is not associated with risks for the newborn and can lead to changes in lifestyle that imply long-term benefits.
Publication types
- Systematic Review
- Delivery, Obstetric
- Exercise / physiology*
- Fetal Development
- Guidelines as Topic
- Labor, Obstetric / physiology
- Pregnancy / physiology*
- Pregnancy Outcome

COMMENTS
Jul 16, 2022 · Our findings reveal that past research on PA in pregnancy focused mostly on health outcomes for the pregnant women and infants. Similar to Evenson et al. (2014), our analysis revealed heterogeneity of findings in terms of exercise modality, duration, and intensity.
These findings were not confirmed in larger prospective epidemiologic studies which found, overall, either neutral or beneficial effects of exercise during pregnancy on birth weight (Hatch et al., 1993; Schramm, Stockbauer, & Hoffman, 1996; Sternfeld, Quesenberry, Eskenazi, & Newman, 1995).
In addition, studies of physical activity during pregnancy that evaluate pregnancy outcomes have found reduced risks of preterm birth, preeclampsia, and gestational diabetes and improved mental health among individuals who regularly engage in physical activity.
Apr 17, 2023 · Exercise contributes significantly to maternal and fetal wellbeing during pregnancy. Traditionally women were advised to refrain from exercise during pregnancy, but newer evidence has shown this to be false. Theoretically, there were concerns about premature labor and the risks of delivering smaller infants to women who exercised during pregnancy.
Nov 17, 2018 · Research has supported exercise during pregnancy as an effective intervention to prevent excessive gestational weight gain. 32 Furthermore, exercise during pregnancy has been identified as an effective approach to control blood sugars to help prevent and manage GDM. 33 Previous studies carried out with pregnant women however have conducted physi...
Benefits of exercise in pregnancy include reduction in Cesarean section rates, appropriate maternal and fetal weight gain, and managing gestational diabetes. Exercise as a means of preventing gestational diabetes, preeclampsia, or perinatal depression cannot be reliably supported.
The major findings were categorized into the following: (a) exercise patterns, (b) demographic correlates/predictors, (c) the influence of pre-pregnancy exercise on pregnancy exercise, (d) theory-based predictors and (f) other correlates of exercise (e.g. general health and safety concerns).
Recent findings: Exercises during pregnancy are associated with higher cardiorespiratory fitness, prevention of urinary incontinence and low back pain, reduced symptoms of depression, gestational weight gain control, and for cases of gestational diabetes, reduced number of women who required insulin. There is no association with reduction in ...
Jan 1, 2023 · In addition, studies of physical activity during pregnancy that evaluate pregnancy outcomes have found reduced risks of preterm birth, preeclampsia, and gestational diabetes mellitus and improved mental health among individuals who regularly engage in physical activity.
Mar 4, 2021 · Future studies should investigate the potential benefits of exercise on uterine contractility and explore what forms of physical activity improve labor outcomes to identify domain-specific interventions during pregnancy.