- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- New advances in type 1...
New advances in type 1 diabetes
- Related content
- Peer review
This article has a correction. Please see:
- New advances in type 1 diabetes - June 03, 2024
- Savitha Subramanian , professor of medicine ,
- Farah Khan , clinical associate professor of medicine ,
- Irl B Hirsch , professor of medicine
- University of Washington Diabetes Institute, Division of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, WA, USA
- Correspondence to: I B Hirsch ihirsch{at}uw.edu
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to modify disease course and preserve β cell function have expanded our broad understanding of this condition. Biomarkers of type 1 diabetes are detectable months to years before development of overt disease, and three stages of diabetes are now recognized. The advent of continuous glucose monitoring and the newer automated insulin delivery systems have changed the landscape of type 1 diabetes management and are associated with improved glycated hemoglobin and decreased hypoglycemia. Adjunctive therapies such as sodium glucose cotransporter-1 inhibitors and glucagon-like peptide 1 receptor agonists may find use in management in the future. Despite these rapid advances in the field, people living in under-resourced parts of the world struggle to obtain necessities such as insulin, syringes, and blood glucose monitoring essential for managing this condition. This review covers recent developments in diagnosis and treatment and future directions in the broad field of type 1 diabetes.
Introduction
Type 1 diabetes is an autoimmune condition that occurs as a result of destruction of the insulin producing β cells of the pancreatic islets, usually leading to severe endogenous insulin deficiency. 1 Without treatment, diabetic ketoacidosis will develop and eventually death will follow; thus, lifelong insulin therapy is needed for survival. Type 1 diabetes represents 5-10% of all diabetes, and diagnosis classically occurs in children but can also occur in adulthood. The burden of type 1 diabetes is expansive; it can result in long term complications, decreased life expectancy, and reduced quality of life and can add significant financial burden. Despite vast improvements in insulin, insulin delivery, and glucose monitoring technology, a large proportion of people with type 1 diabetes do not achieve glycemic goals. The massive burden of type 1 diabetes for patients and their families needs to be appreciated. The calculation and timing of prandial insulin dosing, often from food with unknown carbohydrate content, appropriate food and insulin dosing when exercising, and cost of therapy are all major challenges. The psychological realities of both acute management and the prospect of chronic complications add to the burden. Education programs and consistent surveillance for “diabetes burnout” are ideally available to everyone with type 1 diabetes.
In this review, we discuss recent developments in the rapidly changing landscape of type 1 diabetes and highlight aspects of current epidemiology and advances in diagnosis, technology, and management. We do not cover the breadth of complications of diabetes or certain unique scenarios including psychosocial aspects of type 1 diabetes management, management aspects specific to older adults, and β cell replacement therapies. Our review is intended for the clinical reader, including general internists, family practitioners, and endocrinologists, but we acknowledge the critical role that people living with type 1 diabetes and their families play in the ongoing efforts to understand this lifelong condition.
Sources and selection criteria
We did individual searches for studies on PubMed by using terms relevant to the specific topics covered in this review pertaining to type 1 diabetes. Search terms used included “type 1 diabetes” and each individual topic—diagnosis, autoantibodies, adjuvant therapies, continuous glucose monitoring, automated insulin delivery, immunotherapies, diabetic ketoacidosis, hypoglycemia, and under-resourced settings. We considered all studies published in the English language between 1 January 2001 and 31 January 2023. We selected publications outside of this timeline on the basis of relevance to each topic. We also supplemented our search strategy by a hand search of the references of key articles. We prioritized studies on each highlighted topic according to the level of evidence (randomized controlled trials (RCTs), systematic reviews and meta-analyses, consensus statements, and high quality observational studies), study size (we prioritized studies with at least 50 participants when available), and time of publication (we prioritized studies published since 2003 except for the landmark Diabetes Control and Complications Trial and a historical paper by Tuomi on diabetes autoantibodies, both from 1993). For topics on which evidence from RCTs was unavailable, we included other study types of the highest level of evidence available. To cover all important clinical aspects of the broad array of topics covered in this review, we included additional publications such as clinical reviews as appropriate on the basis of clinical relevance to both patients and clinicians in our opinion.
Epidemiology
The incidence of type 1 diabetes is rising worldwide, possibly owing to epigenetic and environmental factors. Globally in 2020 an estimated 8.7 million people were living with type 1 diabetes, of whom approximately 1.5 million were under 20 years of age. 2 This number is expected to rise to more than 17 million by 2040 ( https://www.t1dindex.org/#global ). The International Diabetes Federation estimates the global prevalence of type 1 diabetes at 0.1%, and this is likely an underestimation as diagnoses of type 1 diabetes in adults are often not accounted for. The incidence of adult onset type 1 diabetes is higher in Europe, especially in Nordic countries, and lowest in Asian countries. 3 Adult onset type 1 diabetes is also more prevalent in men than in women. An increase in prevalence in people under 20 years of age has been observed in several western cohorts including the US, 4 5 Netherlands, 6 Canada, 7 Hungary, 8 and Germany. 9
Classically, type 1 diabetes presents over the course of days or weeks in children and adolescents with polyuria, polydipsia, and weight loss due to glycosuria. The diagnosis is usually straightforward, with profound hyperglycemia (often >300 mg/dL) usually with ketonuria with or without ketoacidemia. Usually, more than one autoantibody is present at diagnosis ( table 1 ). 10 The number of islet autoantibodies combined with parameters of glucose tolerance now forms the basis of risk prediction for type 1 diabetes, with stage 3 being clinical disease ( fig 1 ). 11 The originally discovered autoantibody, islet cell antibody, is no longer used clinically owing to variability of the assay despite standardisation. 12
Autoantibody characteristics associated with increased risk of type 1 diabetes 10
- View inline

Natural history of type 1 diabetes. Adapted with permission from Insel RA, et al. Diabetes Care 2015;38:1964-74 11
- Download figure
- Open in new tab
- Download powerpoint
Half of all new cases of type 1 diabetes are now recognized as occurring in adults. 13 Misclassification due to misdiagnosis (commonly as type 2 diabetes) occurs in nearly 40% of people. 14 As opposed to typical childhood onset type 1 diabetes, progression to severe insulin deficiency, and therefore its clinical presentation in adults, is variable. The term latent autoimmune diabetes of adults (LADA) was introduced 30 years ago to identify adults who developed immune mediated diabetes. 15 An international consensus defined the diagnostic criteria for LADA as age >30 years, lack of need for insulin use for at least six months, and presence of islet cell autoantibodies. 16 However, debate as to whether the term LADA should even be used as a diagnostic term persists. The American Diabetes Association (ADA) Standards of Care note that for the purpose of classification, all forms of diabetes mediated by autoimmune β cell destruction are included in the classification of type 1 diabetes. 17 Nevertheless, they note that use of the term LADA is acceptable owing to the practical effect of heightening awareness of adults likely to have progressive autoimmune β cell destruction and thereby accelerating insulin initiation by clinicians to prevent diabetic ketoacidosis.
The investigation of adults with suspected type 1 diabetes is not always straightforward ( fig 2 ). 18 Islet cell autoantibodies such as glutamic acid decarboxylase antibody (GADA), tyrosine phosphatase IA2 antibody, and zinc transporter isoform 8 autoantibody act as markers of immune activity and can be detected in the blood with standardized assays ( table 1 ). The presence of one or more antibodies in adults with diabetes could mark the progression to severe insulin deficiency; these individuals should be considered to have type 1 diabetes. 1 Autoantibodies, especially GADA, should be measured only in people with clinically suspected type 1 diabetes, as low concentrations of GADA can be seen in type 2 diabetes and thus false positive measurements are a concern. 19 That 5-10% of cases of type 1 diabetes may occur without diabetes autoantibodies is also now clear, 20 and that the diabetes autoantibodies disappear over time is also well appreciated. 21

Flowchart for investigation of suspected type 1 diabetes in adults, based on data from white European populations. No single clinical feature in isolation confirms type 1 diabetes. The most discriminative feature is younger age at diagnosis (<35 years), with lower body mass index (<25), unintentional weight loss, ketoacidosis, and glucose >360 mg/dL at presentation. Adapted with permission from Holt RIG, et al. Diabetes Care 2021;44:2589-625 1
Genetic risk scoring (GRS) for type 1 diabetes has received attention to differentiate people whose classification is unclear. 22 23 24 Developed in 2019, the T1D-GRS2 uses 67 single nucleotide polymorphisms from known autoimmune loci and can predict type 1 diabetes in children of European and African ancestry. Although GRS is not available for routine clinical use, it may allow prediction of future cases of type 1 diabetes to allow prevention strategies with immune intervention (see below).
A major change in the type 1 diabetes phenotype has occurred over the past few decades, with an increase in obesity; the reasons for this are complex. In the general population, including people with type 1 diabetes, an epidemic of sedentary lifestyles and the “westernized diet” consisting of increased processed foods, refined sugars, and saturated fat is occurring. In people with type 1 diabetes, the overall improvement in glycemic control since the report of the Diabetes Control and Complications Trial (DCCT) in 1993 (when one or two insulin injections a day was standard therapy) has resulted in less glycosuria so that the typical patient with lower body weight is uncommon in high income countries. In the US T1D Exchange, more than two thirds of the adult population were overweight or obese. 25
Similarly, obesity in young people with type 1 diabetes has also increased over the decades. 26 The combination of autoimmune insulin deficiency with obesity and insulin resistance has received several descriptive names over the years, with this phenotype being described as double diabetes and hybrid diabetes, among others, 26 27 but no formal nomenclature in the diabetes classification exists. Many of these patients have family members with type 2 diabetes, and some patients probably do have both types of diabetes. Clinically, minimal research has been done into how this specific population responds to certain antihyperglycemic oral agents, such as glucagon-like peptide 1 (GLP-1) receptor agonists, given the glycemic, weight loss, and cardiovascular benefits seen with these agents. 28 These patients are common in most adult diabetes practices, and weight management in the presence of insulin resistance and insulin deficiency remains unclear.
Advances in monitoring
The introduction of home blood glucose monitoring (BGM) more than 45 years ago was met with much skepticism until the report of the DCCT. 29 Since then, home BGM has improved in accuracy, precision, and ease of use. 30 Today, in many parts of the world, home BGM, a static measurement of blood glucose, has been replaced by continuous glucose monitoring (CGM), a dynamic view of glycemia. CGM is superior to home BGM for glycemic control, as confirmed in a meta-analysis of 21 studies and 2149 participants with type 1 diabetes in which CGM use significantly decreased glycated hemoglobin (HbA 1c ) concentrations compared with BGM (mean difference −0.23%, 95% confidence interval −3.83 to −1.08; P<0.001), with a greater benefit if baseline HbA 1c was >8% (mean difference −0.43%, −6.04 to −3.30; P<0.001). 31 This newer technology has also evolved into a critical component of automated insulin delivery. 32
CGM is the standard for glucose monitoring for most adults with type 1 diabetes. 1 This technology uses interstitial fluid glucose concentrations to estimate blood glucose. Two types of CGM are available. The first type, called “real time CGM”, provides a continuous stream of glucose data to a receiver, mobile application, smartwatch, or pump. The second type, “intermittently scanned CGM,” needs to be scanned by a reader device or smartphone. Both of these technologies have shown improvements in HbA 1c and amount of time spent in the hypoglycemic range compared with home BGM when used in conjunction with multiple daily injections or “open loop” insulin pump therapy. 33 34 Real time CGM has also been shown to reduce hypoglycemic burden in older adults with type 1 diabetes ( table 2 ). 36 Alerts that predict or alarm with both hypoglycemia and hyperglycemia can be customized for the patient’s situation (for example, a person with unawareness of hypoglycemia would have an alert at a higher glucose concentration). Family members can also remotely monitor glycemia and be alerted when appropriate. The accuracy of these devices has improved since their introduction in 2006, so that currently available sensors can be used without a confirmation glucose concentration to make a treatment decision with insulin. However, some situations require home BGM, especially when concerns exist that the CGM does not match symptoms of hypoglycemia.
Summary of trials for each topic covered
Analysis of CGM reports retrospectively can assist therapeutic decision making both for the provider and the patient. Importantly, assessing the retrospective reports and watching the CGM in real time together offer insight to the patient with regard to insulin dosing, food choices, and exercise. Patients should be encouraged to assess their data on a regular basis to better understand their diabetes self-management. Table 3 shows standard metrics and targets for CGM data. 52 Figure 3 shows an ambulatory glucose profile.
Standardized continuous glucose monitoring metrics for adults with diabetes 52

Example of ambulatory glucose profile of 52 year old woman with type 1 diabetes and fear of hypoglycemia. CGM=continuous glucose monitoring; GMI=glucose management indicator
Improvements in technology and evidence for CGM resulting in international recommendations for its widespread use have resulted in greater uptake by people with type 1 diabetes across the globe where available and accessible. Despite this, not everyone wishes to use it; some people find wearing any device too intrusive, and for many the cost is prohibitive. These people need at the very least before meal and bedtime home BGM.
A next generation implantable CGM device (Sensionics), with an improved calibration algorithm that lasts 180 days after insertion by a healthcare professional, is available in both the EU and US. Although fingerstick glucose calibration is needed, the accuracy is comparable to that of other available devices. 53
Advances in treatments
The discovery of insulin in 1921, resulting in a Nobel Prize, was considered one of the greatest scientific achievements of the 20th century. The development of purified animal insulins in the late 1970s, followed by human insulin in the early 1980s, resulted in dramatic reductions in allergic reactions and lipoatrophy. Introduction of the first generation of insulin analogs, insulin lispro in the mid-1990s followed by insulin glargine in the early 2000s, was an important advance for the treatment of type 1 diabetes. 54 We review the next generation of insulin analogs here. Table 4 provides details on available insulins.
Pharmacokinetics of commonly used insulin preparations
Ultra-long acting basal insulins
Insulin degludec was developed with the intention of improving the duration of action and achieving a flatter profile compared with the original long acting insulin analogs, insulin glargine and insulin detemir. Its duration of action of 42 hours at steady state means that the profile is generally flat without significant day-to-day variability, resulting in less hypoglycemia compared with U-100 glargine. 39 55
When U-100 insulin glargine is concentrated threefold, its action is prolonged. 56 U-300 glargine has a different kinetic profile and is delivered in one third of the volume of U-100 glargine, with longer and flatter effects. The smaller volume of U-300 glargine results in slower and more gradual release of insulin monomers owing to reduced surface area in the subcutaneous space. 57 U-300 glargine also results in lesser hypoglycemia compared with U-100 glargine. 58
Ultra-rapid acting prandial insulins
Rapid acting insulin analogs include insulin lispro, aspart, and glulisine. With availability of insulin lispro, the hope was for a prandial insulin that better matched food absorption. However, these newer insulins are too slow to control the glucose spike seen with ingestion of a high carbohydrate load, leading to the development of insulins with even faster onset of action.
The first available ultra-rapid prandial insulin was fast acting insulin aspart. This insulin has an onset of appearance approximately twice as fast (~5 min earlier) as insulin aspart, whereas dose-concentration and dose-response relations are comparable between the two insulins ( table 4 ). 59 In adults with type 1 diabetes, mealtime and post-meal fast acting aspart led to non-inferior glycemic control compared with mealtime aspart, in combination with basal insulin. 60 Mean HbA 1c was 7.3%, 7.3%, and 7.4% in the mealtime faster aspart, mealtime aspart, and post‐meal faster aspart arms, respectively (P<0.001 for non-inferiority).
Insulin lispro-aabc is the second ultra-rapid prandial insulin. In early kinetic studies, insulin lispro-aabc appeared in the serum five minutes faster with 6.4-fold greater exposure in the first 15 minutes compared with insulin lispro. 61 The duration of exposure of the insulin concentrations in this study was 51 minutes faster with lispro-aabc. Overall insulin exposure was similar between the two groups. Clinically, lispro-aabc is non-inferior to insulin lispro, but postprandial hyperglycemia is lower with the faster acting analog. 62 Lispro-aabc given at mealtime resulted in greater improvement in post-prandial glucose (two hour post-prandial glucose −31.1 mg/dL, 95% confidence interval −41.0 to −21.2; P<0.001).
Both ultra-rapid acting insulins can be used in insulin pumps. Lispro-aabc tends to have more insertion site reactions than insulin lispro. 63 A meta-analysis including nine studies and 1156 participants reported increased infusion set changes on rapid acting insulin analogs (odds ratio 1.60, 95% confidence interval 1.26 to 2.03). 64
Pulmonary inhaled insulin
The quickest acting insulin is pulmonary inhaled insulin, with an onset of action of 12 minutes and a duration of 1.5-3 hours. 65 When used with postprandial supplemental dosing, glucose control is improved without an increase in hypoglycemia. 66
Insulin delivery systems
Approved automated insulin delivery systems.
CGM systems and insulin pumps have shown improvement in glycemic control and decreased risk of severe hypoglycemia compared with use of self-monitoring of blood glucose and multiple daily insulin injections in type 1 diabetes. 67 68 69 Using CGM and insulin pump together (referred to as sensor augmented pump therapy) only modestly improves HbA 1c in patients who have high sensor wear time, 70 71 but the management burden of diabetes does not decrease as frequent user input is necessary. Thus emerged the concept of glucose responsive automated insulin delivery (AID), in which data from CGM can inform and allow adjustment of insulin delivery.
In the past decade, exponential improvements in CGM technologies and refined insulin dosing pump algorithms have led to the development of AID systems that allow for minimization of insulin delivery burden. The early AID systems reduced hypoglycemia risk by automatically suspending insulin delivery when glucose concentrations dropped to below a pre-specified threshold but did not account for high glucose concentrations. More complex algorithms adjusting insulin delivery up and down automatically in response to real time sensor glucose concentrations now allow close replication of normal endocrine pancreatic physiology.
AID systems (also called closed loop or artificial pancreas systems) include three components—an insulin pump that continuously delivers rapid acting insulin, a continuous glucose sensor that measures interstitial fluid glucose at frequent intervals, and a control algorithm that continuously adjusts insulin delivery that resides in the insulin pump or a smartphone application or handheld device ( fig 4 ). All AID systems that are available today are referred to as “hybrid” closed loop (HCL) systems, as users are required to manually enter prandial insulin boluses and signal exercise, but insulin delivery is automated at night time and between meals. AID systems, regardless of the type used, have shown benefit in glycemic control and cost effectiveness, improve quality of life by improving sleep quality, and decrease anxiety and diabetes burden in adults and children. 72 73 74 Limitations to today’s HCL systems are primarily related to pharmacokinetics and pharmacodynamics of available analog insulins and accuracy of CGM in extremes of blood glucose values. The iLet bionic pancreas, cleared by the US Food and Drug Administration (FDA) in May 2023, is an AID system that determines all therapeutic insulin doses for an individual on the basis of body weight, eliminating the need for calculation of basal rates, insulin to carbohydrate ratios, blood glucose corrections, and bolus dose. The control algorithms adapt continuously and autonomously to the individual’s insulin needs. 38 Table 5 lists available AID systems.

Schematic of closed loop insulin pump technology. The continuous glucose monitor senses interstitial glucose concentrations and sends the information via Bluetooth to a control algorithm hosted on an insulin pump (or smartphone). The algorithm calculates the amount of insulin required, and the insulin pump delivers rapid acting insulin subcutaneously
Comparison of commercially available hybrid closed loop systems 75
Unapproved systems
Do-it-yourself (DIY) closed loop systems—DIY open artificial pancreas systems—have been developed by people with type 1 diabetes with the goal of self-adjusting insulin by modifying their individually owned devices. 76 These systems are built by the individual using an open source code widely available to anyone with compatible medical devices who is willing and able to build their own system. DIY systems are used by several thousand people across the globe but are not approved by regulatory bodies; they are patient-driven and considered “off-label” use of technology with the patient assuming full responsibility for their use. Clinicians caring for these patients should ensure basic diabetes skills, including pump site maintenance, a knowledge of how the chosen system works, and knowing when to switch to “manual mode” for patients using an artificial pancreas system of any kind. 76 The small body of studies on DIY looping suggests improvement in HbA 1c , increased time in range, decreased hypoglycemia and glucose variability, improvement in night time blood glucose concentrations, and reduced mental burden of diabetes management. 77 78 79 Although actively prescribing or initiating these options is not recommended, these patients should be supported by clinical teams; insulin prescription should not be withheld, and, if initiated by the patient, unregulated DIY options should be openly discussed to ensure open and transparent relationships. 78
In January 2023, the US FDA cleared the Tidepool Loop app, a DIY AID system. This software will connect the CGM, insulin pump, and Loop algorithm, but no RCTs using this method are available.
β cell replacement therapies
For patients with type 1 diabetes who meet specific clinical criteria, β cell replacement therapy using whole pancreas or pancreatic islet transplantation can be considered. Benefits of transplantation include immediate cessation of insulin therapy, attainment of euglycemia, and avoidance of hypoglycemia. Additional benefits include improved quality of life and stabilization of complications. 80 Chronic immunosuppression is needed to prevent graft rejection after transplantation.
Pancreas transplantation
Whole pancreas transplantation, first performed in 1966, involves complex abdominal surgery and lifelong immunosuppressive therapy and is limited by organ donor availability. Today, pancreas transplants are usually performed simultaneously using two organs from the same donor (simultaneous pancreas-kidney transplant (SPKT)), sequentially if the candidate has a living donor for renal transplantation (pancreas after kidney transplant (PAKT)) or on its own (pancreas transplantation alone). Most whole pancreas transplants are performed with kidney transplantation for end stage diabetic kidney disease. Pancreas graft survival at five years after SPKT is 80% and is superior to that with pancreas transplants alone (62%) or PAKT (67%). 81 Studies from large centers where SPKT is performed show that recipients can expect metabolic improvements including amelioration of problematic hypoglycemia for at least five years. 81 The number of pancreas transplantations has steadily decreased in the past two decades.
Islet transplantation
Islet transplantation can be pursued in selected patients with type 1 diabetes marked by unawareness of hypoglycemia and severe hypoglycemic episodes, to help restore the α cell response critical for responding to hypoglycemia. 82 83 Islet transplantation involves donor pancreas procurement with subsequent steps to isolate, purify, culture, and infuse the islets. Multiple donors are needed to provide enough islet cells to overcome islet cell loss during transplantation. Survival of the islet grafts, limited donor supply, and lifelong need for immunosuppressant therapy remain some of the biggest challenges. 84 Islet transplantation remains experimental in the US and is offered in a few specialized centers in North America, some parts of Europe, and Australia. 85
Disease modifying treatments for β cell preservation
Therapies targeting T cells, B cells, and cytokines that find use in a variety of autoimmune diseases have also been applied to type 1 diabetes. The overarching goal of immune therapies in type 1 diabetes is to prevent or delay the loss of functional β cell mass. Studies thus far in early type 1 diabetes have not yet successfully shown reversal of loss of C peptide or maintenance of concentrations after diagnosis, although some have shown preservation or slowing of loss of β cells. This suggests that a critical time window of opportunity exists for starting treatment depending on the stage of type 1 diabetes ( fig 1 ).
Teplizumab is a humanized monoclonal antibody against the CD3 molecule on T cells; it is thought to modify CD8 positive T lymphocytes, key effector cells that mediate β cell death and preserves regulatory T cells. 86 Teplizumab, when administered to patients with new onset of type 1 diabetes, was unable to restore glycemia despite C peptide preservation. 87 However, in its phase II prevention study of early intervention in susceptible individuals (at least two positive autoantibodies and an abnormal oral glucose tolerance test at trial entry), a single course of teplizumab delayed progression to clinical type 1 diabetes by about two years ( table 2 ). 43 On the basis of these results, teplizumab received approval in the US for people at high risk of type 1 diabetes in November 2022. 88 A phase III trial (PROTECT; NCT03875729 ) to evaluate the efficacy and safety of teplizumab versus placebo in children and adolescents with new diagnosis of type 1 diabetes (within six weeks) is ongoing. 89
Thus far, targeting various components of the immune response has been attempted in early type 1 diabetes without any long term beneficial effects on C peptide preservation. Co-stimulation blockade using CTLA4-Ig abatacept, a fusion protein that interferes with co-stimulation needed in the early phases of T cell activation that occurs in type 1 diabetes, is being tested for efficacy in prevention of type 1 diabetes ( NCT01773707 ). 90 Similarly, several cytokine directed anti-inflammatory targets (interleukin 6 receptor, interleukin 1β, tumor necrosis factor ɑ) have not shown any benefit.
Non-immunomodulatory adjunctive therapies
Adjunctive therapies for type 1 diabetes have been long entertained owing to problems surrounding insulin delivery, adequacy of glycemic management, and side effects associated with insulin, especially weight gain and hypoglycemia. At least 50% of adults with type 1 diabetes are overweight or obese, presenting an unmet need for weight management in these people. Increased cardiovascular risk in these people despite good glycemic management presents additional challenges. Thus, use of adjuvant therapies may tackle these problems.
Metformin, by decreasing hepatic glucose production, could potentially decrease fasting glucose concentrations. 91 It has shown benefit in reducing insulin doses and possibly improving metabolic control in obese/overweight people with type 1 diabetes. A meta-analysis of 19 RCTs suggests short term improvement in HbA 1c that is not sustained after three months and is associated with higher incidence of gastrointestinal side effects. 92 No evidence shows that metformin decreases cardiovascular morbidity in type 1 diabetes. Therefore, owing to lack of conclusive benefit, addition of metformin to treatment regimens is not recommended in consensus guidelines.
Glucagon-like peptide receptor agonists
Endogenous GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient ingestion and enhances glucose induced insulin secretion, suppresses glucagon secretion, delays gastric emptying, and induces satiety. 93 GLP-1 promotes β cell proliferation and inhibits apoptosis, leading to expansion of β cell mass. GLP-1 secretion in patients with type 1 diabetes is similar to that seen in people without diabetes. Early RCTs of liraglutide in type 1 diabetes resulted in weight loss and modest lowering of HbA 1c ( table 2 ). 49 50 Liraglutide 1.8 mg in people with type 1 diabetes and higher body mass index decreased HbA 1c , weight, and insulin requirements with no increased hypoglycemia risk. 94 However, on the basis of results from a study of weekly exenatide that showed similar results, these effects may not be sustained. 51 A meta-analysis of 24 studies including 3377 participants showed that the average HbA 1c decrease from GLP-1 receptor agonists compared with placebo was highest for liraglutide 1.8 mg daily (−0.28%, 95% confidence interval −0.38% to−0.19%) and exenatide (−0.17%, −0.28% to 0.02%). The estimated weight loss from GLP-1 receptor agonists compared with placebo was −4.89 (−5.33 to−4.45) kg for liraglutide 1.8 mg and −4.06 (−5.33 to−2.79) kg for exenatide. 95 No increase in severe hypoglycemia was seen (odds ratio 0.67, 0.43 to 1.04) but therapy was associated with higher levels of nausea. GLP-1 receptor agonist use may be beneficial for weight loss and reducing insulin doses in a subset of patients with type 1 diabetes. GLP-1 receptor agonists are not a recommended treatment option in type 1 diabetes. Semaglutide is being studied in type 1 diabetes in two clinical trials ( NCT05819138 ; NCT05822609 ).
Sodium-glucose cotransporter inhibitors
Sodium-glucose cotransporter 2 (SGLT-2), a protein expressed in the proximal convoluted tubule of the kidney, reabsorbs filtered glucose; its inhibition prevents glucose reabsorption in the tubule and increases glucose excretion by the kidney. Notably, the action of these agents is independent of insulin, so this class of drugs has potential as adjunctive therapy for type 1 diabetes. Clinical trials have shown significant benefit in cardiovascular and renal outcomes in type 2 diabetes; therefore, significant interest exists for use in type 1 diabetes. Several available SGLT-2 inhibitors have been studied in type 1 diabetes and have shown promising results with evidence of decreased total daily insulin dosage, improvement in HbA 1c , lower rates of hypoglycemia, and decrease in body weight; however, these effects do not seem to be sustained at one year in clinical trials and seem to wane with time. Despite beneficial effects, increased incidence of diabetic ketoacidosis has been observed in all trials, is a major concern, and is persistent despite educational efforts. 96 97 98 Low dose empagliflozin (2.5 mg) has shown lower rates of diabetic ketoacidosis in clinical trials ( table 2 ). 47 Favorable risk profiles have been noted in Japan, the only market where SGLT-2 inhibitors are approved for adjunctive use in type 1 diabetes. 99 In the US, SGLT-2 inhibitors are approved for use in type 2 diabetes only. In Europe, although dapagliflozin was approved for use as adjunct therapy to insulin in adults with type 1 diabetes, the manufacturer voluntarily withdrew the indication for the drug in 2021. 100 Sotagliflozin is a dual SGLT-1 and SGLT-2 inhibitor that decreases renal glucose reabsorption through systemic inhibition of SGLT-2 and decreases glucose absorption in the proximal intestine by SGLT-1 inhibition, blunting and delaying postprandial hyperglycemia. 101 Studies of sotagliflozin in type 1 diabetes have shown sustained HbA 1c reduction, weight loss, lower insulin requirements, lesser hypoglycemia, and more diabetic ketoacidosis relative to placebo. 102 103 104 The drug received authorization in the EU for use in type 1 diabetes, but it is not marketed there. Although SGLT inhibitors are efficacious in type 1 diabetes management, the risk of diabetic ketoacidosis is a major limitation to widespread use of these agents.
Updates in acute complications of type 1 diabetes
Diabetic ketoacidosis.
Diabetic ketoacidosis is a serious and potentially fatal hyperglycemic emergency accompanied by significant rates of mortality and morbidity as well as high financial burden for healthcare systems and societies. In the past decade, increasing rates of diabetic ketoacidosis in adults have been observed in the US and Europe. 105 106 This may be related to changes in the definition of diabetic ketoacidosis, use of medications associated with higher risk, and admission of patients at lower risk. 107 In a US report of hospital admissions with diabetic ketoacidosis, 53% of those admitted were between the ages of 18 and 44, with higher rates in men than in women. 108 Overall, although mortality from diabetic ketoacidosis in developed countries remains low, rates have risen in people aged >60 and in those with coexisting life threatening illnesses. 109 110 Recurrent diabetic ketoacidosis is associated with a substantial mortality rate. 111 Frequency of diabetic ketoacidosis increases with higher HbA 1c concentrations and with lower socioeconomic status. 112 Common precipitating factors include newly diagnosed type 1 diabetes, infection, poor adherence to insulin, and an acute cardiovascular event. 109
Euglycemic diabetic ketoacidosis refers to the clinical picture of an increased anion gap metabolic acidosis, ketonemia, or significant ketonuria in a person with diabetes without significant glucose elevation. This can be seen with concomitant use of SGLT-2 inhibitors (currently not indicated in type 1 diabetes), heavy alcohol use, cocaine use, pancreatitis, sepsis, and chronic liver disease and in pregnancy 113 Treatment is similar to that for hyperglycemic diabetic ketoacidosis but can require earlier use and greater concentrations of a dextrose containing fluid for the insulin infusion in addition to 0.9% normal saline resuscitation fluid. 114
The diagnosis of diabetic ketoacidosis has evolved from a gluco-centric diagnosis to one requiring hyperketonemia. By definition, independent of blood glucose, a β-hydroxybutyrate concentration >3 mmol/L is required for diagnosis. 115 However, the use of this ketone for assessment of the severity of the diabetic ketoacidosis is controversial. 116 Bedside β-hydroxybutyrate testing during treatment is standard of care in many parts of the world (such as the UK) but not others (such as the US). Concerns have been raised about accuracy of bedside β-hydroxybutyrate meters, but this is related to concentrations above the threshold for diabetic ketoacidosis. 116
Goals for management of diabetic ketoacidosis include restoration of circulatory volume, correction of electrolyte imbalances, and treatment of hyperglycemia. Intravenous regular insulin infusion is the standard of care for treatment worldwide owing to rapidity of onset of action and rapid resolution of ketonemia and hyperglycemia. As hypoglycemia and hypokalemia are more common during treatment, insulin doses are now recommended to be reduced from 0.1 u/kg/h to 0.05 u/kg/h when glucose concentrations drop below 250 mg/dL or 14 mM. 115 Subcutaneous rapid acting insulin protocols have emerged as alternative treatments for mild to moderate diabetic ketoacidosis. 117 Such regimens seem to be safe and have the advantages of not requiring admission to intensive care, having lower rates of complications related to intravenous therapy, and requiring fewer resources. 117 118 Ketonemia and acidosis resolve within 24 hours in most people. 115 To prevent rebound hyperglycemia, the transition off an intravenous insulin drip must overlap subcutaneous insulin by at least two to four hours. 115
Hypoglycemia
Hypoglycemia, a common occurrence in people with type 1 diabetes, is a well appreciated effect of insulin treatment and occurs when blood glucose falls below the normal range. Increased susceptibility to hypoglycemia from exogenous insulin use in people with type 1 diabetes results from multiple factors, including imperfect subcutaneous insulin delivery tools, loss of glucagon within a few years of diagnosis, progressive impairment of the sympatho-adrenal response with repeated hypoglycemic episodes, and eventual development of impaired awareness. In 2017 the International Hypoglycemia Study Group developed guidance for definitions of hypoglycemia; on the basis of this, a glucose concentration of 3.0-3.9 mmol/L (54-70 mg/dL) was designated as level 1 hypoglycemia, signifying impending development of level 2 hypoglycemia—a glucose concentration <3 mmol/L (54 mg/dL). 119 120 At approximately 54 mg/dL, neuroglycopenic hypoglycemia symptoms, including vision and behavior changes, seizures, and loss of consciousness, begin to occur as a result of glucose deprivation of neurons in the central nervous system. This can eventually lead to cerebral dysfunction at concentrations <50 mg/dL. 121 Severe hypoglycemia (level 3), denoting severe cognitive and/or physical impairment and needing external assistance for recovery, is a common reason for emergency department visits and is more likely to occur in people with lower socioeconomic status and with the longest duration of diabetes. 112 Prevalence of self-reported severe hypoglycemia is very high according to a global population study that included more than 8000 people with type 1 diabetes. 122 Severe hypoglycemia occurred commonly in younger people with suboptimal glycemia according to a large electronic health record database study in the US. 123 Self- reported severe hypoglycemia is associated with a 3.4-fold increase in mortality. 124 125
Acute consequences of hypoglycemia include impaired cognitive function, temporary focal deficits including stroke-like symptoms, and memory deficits. 126 Cardiovascular effects including tachycardia, arrhythmias, QT prolongation, and bradycardia can occur. 127 Hypoglycemia can impair many activities of daily living, including motor vehicle safety. 128 In a survey of adults with type 1 diabetes who drive a vehicle at least once a week, 72% of respondents reported having hypoglycemia while driving, with around 5% reporting a motor vehicle accident due to hypoglycemia in the previous two years. 129 This contributes to the stress and fear that many patients face while grappling with the difficulties of ongoing hypoglycemia. 130
Glucagon is highly efficacious for the primary treatment of severe hypoglycemia when a patient is unable to ingest carbohydrate safely, but it is unfortunately under-prescribed and underused. 131 132 Availability of nasal, ready to inject, and shelf-stable liquid glucagon formulations have superseded the need for reconstituting older injectable glucagon preparations before administration and are now preferred. 133 134 Real time CGM studies have shown a decreased hypoglycemic exposure in people with impaired awareness without a change in HbA 1c . 34 135 136 137 138 CGM has shown benefit in decreasing hypoglycemia across the lifespan, including in teens, young adults, and older people. 36 139 Although CGM reduces the burden of hypoglycemia including severe hypoglycemia, it does not eliminate it; overall, such severe level 3 hypoglycemia rates in clinical trials are very low and hard to decipher in the real world. HCL insulin delivery systems integrated with CGM have been shown to decrease hypoglycemia. Among available rapid acting insulins, ultra-rapid acting lispro (lispro-aabc) seems to be associated with less frequent hypoglycemia in type 1 diabetes. 140 141
As prevention of hypoglycemia is a crucial aspect of diabetes management, formal training programs to increase awareness and education on avoidance of hypoglycemia, such as the UK’s Dose Adjustment for Normal Eating (DAFNE), have been developed. 142 143 This program has shown fewer severe hypoglycemia (mean 1.7 (standard deviation 8.5) episodes per person per year before training to 0.6 (3.7) episodes one year after training) and restoration of recognition of hypoglycemia in 43% of people reporting unawareness. Clinically relevant anxiety and depression fell from 24.4% to 18.0% and from 20.9% to 15.5%, respectively. A structured education program with cognitive and psychotherapeutic aspects for changing hypoglycemia related behaviors, called the Hypoglycemia Awareness Restoration Program despite optimized self-care (HARPdoc), showed a positive effect on changing unhelpful beliefs around hypoglycemia and improved diabetes related and general distress and anxiety scores. 144
Management in under-resourced settings
According to a recent estimate from the International Diabetes Federation, 1.8 million people with type 1 diabetes live in low and middle income countries (LMICs). 2 In many LMICs, the actual burden of type 1 diabetes remains unknown and material resources needed to manage type 1 diabetes are lacking. 145 146 Health systems in these settings are underequipped to tackle the complex chronic disease that is type 1 diabetes. Few diabetes and endocrinology specialist physicians are available owing to lack of specific postgraduate training programs in many LMICs; general practitioners with little to no clinical experience in managing type 1 diabetes care for these patients. 146 This, along with poor availability and affordability of insulin and lack of access to technology, results in high mortality rates. 147 148 149 In developed nations, low socioeconomic status is associated with higher levels of mortality and morbidity for adults with type 1 diabetes despite access to a universal healthcare system. 150 Although global governments have committed to universal health coverage and therefore widespread availability of insulin, it remains very far from realization in most LMICs. 151
Access to technology is patchy and varies globally. In the UST1DX, CGM use was least in the lowest fifth of socioeconomic status. 152 Even where technology is available, successful engagement does not always occur. 153 In a US cohort, lower CGM use was seen in non-Hispanic Black children owing to lower rates of device initiation and higher rates of discontinuation. 154 In many LMICs, blood glucose testing strips are not readily available and cost more than insulin. 151 In resource limited settings, where even diagnosis, basic treatments including insulin, syringes, and diabetes education are limited, use of CGM adds additional burden to patients. Need for support services and the time/resources needed to download and interpret data are limiting factors from a clinician’s perspective. Current rates of CGM use in many LMICs are unknown.
Inequities in the availability of and access to certain insulin formulations continue to plague diabetes care. 155 In developed countries such as the US, rising costs have led to insulin rationing by around 25% of people with type 1 diabetes. 156 LMICs have similar trends while also remaining burdened by disproportionate mortality and complications from type 1 diabetes. 155 157 With the inclusion of long acting insulin analogs in the World Health Organization’s Model List of Essential Medicines in 2021, hope has arisen that these will be included as standard of care across the world. 158 In the past, the pricing of long acting analogs has limited their use in resource poor settings 159 ; however, their inclusion in WHO’s list was a major step in improving their affordability. 158 With the introduction of lower cost long acting insulin biosimilars, improved access to these worldwide in the future can be anticipated. 160
Making insulin available is not enough on its own to improve the prognosis for patients with diabetes in resource poor settings. 161 Improved healthcare infrastructure, better availability of diabetes supplies, and trained personnel are all critical to improving type 1 diabetes care in LMICs. 161 Despite awareness of limitations and barriers, a clear understanding of how to implement management strategies in these settings is still lacking. The Global Diabetes Compact was launched in 2021 with the goal of increasing access to treatment and improving outcomes for people with diabetes across the globe. 162
Emerging technologies and treatments
Monitoring systems.
The ability to measure urinary or more recently blood ketone concentrations is an integral part of self-management of type 1 diabetes, especially during acute illness, intermittent fasting, and religious fasts to prevent diabetic ketoacidosis. 163 Many people with type 1 diabetes do not adhere to urine or blood ketone testing, which likely results in unnecessary episodes of diabetic ketoacidosis. 164 Noting that blood and urine ketone testing is not widely available in all countries and settings is important. 1 Regular assessment of patients’ access to ketone testing (blood or urine) is critical for all clinicians. Euglycemic diabetic ketoacidosis in type 1 diabetes is a particular problem with concomitant use of SGLT-2 inhibitors; for this reason, these agents are not approved for use in these patients. For sick day management (and possibly for the future use of SGLT-2 inhibitors in people with type 1 diabetes), it is hoped that continuous ketone monitoring (CKM) can mitigate the risks of diabetic ketoacidosis. 165 Like CGM, the initial CKM device measures interstitial fluid β-hydroxybutyrate instead of glucose. CKM use becomes important in conjunction with a hybrid closed loop insulin pump system and added SGLT-2 inhibitor therapy, where insulin interruptions are common and hyperketonemia is frequent. 166
Perhaps the greatest technological challenge to date has been the development of non-invasive glucose monitoring. Numerous attempts have been made using strategies including optics, microwave, and electrochemistry. 167 Lack of success to date has resulted in healthy skepticism from the medical community. 168 However, active interest in the development of non-invasive technology with either interstitial or blood glucose remains.
Insulin and delivery systems
In the immediate future, two weekly basal insulins, insulin icodec and basal insulin Fc, may become available. 169 Studies of insulin icodec in type 1 diabetes are ongoing (ONWARDS 6; NCT04848480 ). How these insulins will be incorporated in management of type 1 diabetes is not yet clear.
Currently available AID systems use only a single hormone, insulin. Dual hormone AID systems incorporating glucagon are in development. 170 171 Barriers to the use of dual hormone systems include the need for a second chamber in the pump, a lack of stable glucagon formulations approved for long term subcutaneous delivery, lack of demonstrated long term safety, and gastrointestinal side effects from glucagon use. 74 Similarly, co-formulations of insulin and amylin (a hormone co-secreted with insulin and deficient in people with type 1 diabetes) are in development. 172
Immunotherapy for type 1 diabetes
As our understanding of the immunology of type 1 diabetes expands, development of the next generation of immunotherapies is under active pursuit. Antigen specific therapies, peptide immunotherapy, immune tolerance using DNA vaccination, and regulatory T cell based adoptive transfer targeting β cell senescence are all future opportunities for drug development. Combining immunotherapies with metabolic therapies such as GLP-1 receptor agonists to help to improve β cell mass is being actively investigated.
The quest for β cell replacement methods is ongoing. Transplantation of stem cell derived islets offers promise for personalized regenerative therapies as a potentially curative method that does away with the need for donor tissue. Since the first in vivo model of glucose responsive β cells derived from human embryonic stem cells, 173 different approaches have been attempted. Mesenchymal stromal cell treatment and autologous hematopoietic stem cells in newly diagnosed type 1 diabetes may preserve β cell function without any safety signals. 174 175 176 Stem cell transplantation for type 1 diabetes remains investigational. Encapsulation, in which β cells are protected using a physical barrier to prevent immune attack and avoid lifelong immunosuppression, and gene therapy techniques using CRISPR technology also remain in early stages of investigation.
Until recently, no specific guidelines for management of type 1 diabetes existed and management guidance was combined with consensus statements developed for type 2 diabetes. Table 6 summarizes available guidance and statements from various societies. A consensus report for management of type 1 diabetes in adults by the ADA and European Association for the Study of Diabetes became available in 2021; it covers several topics of diagnosis and management of type 1 diabetes, including glucose monitoring, insulin therapy, and acute complications. Similarly, the National Institute for Health and Care Excellence also offers guidance on management of various aspects of type 1 diabetes. Consensus statements for use of CGM, insulin pump, and AID systems are also available.
Guidelines in type 1 diabetes
Conclusions
Type 1 diabetes is a complex chronic condition with increasing worldwide prevalence affecting several million people. Several successes in management of type 1 diabetes have occurred over the years from the serendipitous discovery of insulin in 1921 to blood glucose monitoring, insulin pumps, transplantation, and immunomodulation. The past two decades have seen advancements in diagnosis, treatment, and technology including development of analog insulins, CGM, and advanced insulin delivery systems. Although we have gained a broad understanding on many important aspects of type 1 diabetes, gaps still exist. Pivotal research continues targeting immune targets to prevent or delay onset of type 1 diabetes. Although insulin is likely the oldest of existing modern drugs, no low priced generic supply of insulin exists anywhere in the world. Management of type 1 diabetes in under resourced areas continues to be a multifaceted problem with social, cultural, and political barriers.
Glossary of abbreviations
ADA—American Diabetes Association
AID—automated insulin delivery
BGM—blood glucose monitoring
CGM—continuous glucose monitoring
CKM—continuous ketone monitoring
DCCT—Diabetes Control and Complications Trial
DIY—do-it-yourself
FDA—Food and Drug Administration
GADA—glutamic acid decarboxylase antibody
GLP-1—glucagon-like peptide 1
GRS—genetic risk scoring
HbA1c—glycated hemoglobin
HCL—hybrid closed loop
LADA—latent autoimmune diabetes of adults
LMIC—low and middle income country
PAKT—pancreas after kidney transplant
RCT—randomized controlled trial
SGLT-2—sodium-glucose cotransporter 2
SPKT—simultaneous pancreas-kidney transplant
Questions for future research
What future new technologies can be helpful in management of type 1 diabetes?
How can newer insulin delivery methods benefit people with type 1 diabetes?
What is the role of disease modifying treatments in prevention and delay of type 1 diabetes?
Is there a role for sodium-glucose co-transporter inhibitors or glucagon-like peptide 1 receptor angonists in the management of type 1 diabetes?
As the population with type 1 diabetes ages, how should management of these people be tailored?
How can we better serve people with type 1 diabetes who live in under-resourced settings with limited access to medications and technology?
How patients were involved in the creation of this manuscript
A person with lived experience of type 1 diabetes reviewed a draft of the manuscript and offered input on important aspects of their experience that should be included. This person is involved in large scale education and activism around type 1 diabetes. They offered their views on various aspects of type 1 diabetes, especially the use of adjuvant therapies and the burden of living with diabetes. This person also raised the importance of education of general practitioners on the various stages of type 1 diabetes and the management aspects. On the basis of this feedback, we have highlighted the burden of living with diabetes on a daily basis.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: SS and IBH contributed to the planning, drafting, and critical review of this manuscript. FNK contributed to the drafting of portions of the manuscript. All three authors are responsible for the overall content as guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: SS has received an honorarium from Abbott Diabetes Care; IBH has received honorariums from Abbott Diabetes Care, Lifescan, embecta, and Hagar and research support from Dexcom and Insulet.
Provenance and peer review: Commissioned; externally peer reviewed.
- DeVries JH ,
- Hess-Fischl A ,
- Gregory GA ,
- Robinson TIG ,
- Linklater SE ,
- International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group
- Harding JL ,
- Wander PL ,
- Dabelea D ,
- Mayer-Davis EJ ,
- SEARCH for Diabetes in Youth Study
- Lawrence JM ,
- SEARCH for Diabetes in Youth Study Group
- Fazeli Farsani S ,
- Souverein PC ,
- van der Vorst MM ,
- Sutherland J ,
- Rokszin G ,
- Stahl-Pehe A ,
- Kamrath C ,
- Atkinson MA ,
- Bingley PJ ,
- Bonifacio E ,
- Leslie RD ,
- Evans-Molina C ,
- Freund-Brown J ,
- Thomas NJ ,
- Zimmet PZ ,
- Rowley MJ ,
- Knowles W ,
- Fourlanos S ,
- Greenbaum CJ ,
- ElSayed NA ,
- American Diabetes Association
- Miller RG ,
- Secrest AM ,
- Sharma RK ,
- Songer TJ ,
- McDonald T ,
- Greenfield JR
- Tridgell DM ,
- Spiekerman C ,
- Greenbaum CJ
- Glessner J ,
- Foster NC ,
- Miller KM ,
- Becker DJ ,
- Khawandanah J
- Pedrosa MR ,
- Franco DR ,
- Gieremek HW ,
- Nathan DM ,
- Diabetes Control and Complications Trial Research Group
- Klonoff DC ,
- Parkes JL ,
- Kovatchev BP ,
- Raghinaru D ,
- iDCL Trial Research Group
- Riddlesworth T ,
- DIAMOND Study Group
- Leelarathna L ,
- Neupane S ,
- FLASH-UK Trial Study Group
- Polonsky W ,
- Hirsch IB ,
- Pratley RE ,
- Kanapka LG ,
- Rickels MR ,
- Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group
- Tauschmann M ,
- APCam11 Consortium
- Russell SJ ,
- Damiano ER ,
- Bionic Pancreas Research Group
- Bailey TS ,
- Group Information ,
- Bergenstal RM ,
- Dellva MA ,
- Mathieu C ,
- Herold KC ,
- Type 1 Diabetes TrialNet Study Group
- Libman IM ,
- DiMeglio LA ,
- T1D Exchange Clinic Network Metformin RCT Study Group
- Petrie JR ,
- Chaturvedi N ,
- REMOVAL Study Group
- Dandona P ,
- Phillip M ,
- DEPICT-1 Investigators
- Rosenstock J ,
- Marquard J ,
- Laffel LM ,
- Hemmingsson JU ,
- ADJUNCT ONE Investigators
- Pieber TR ,
- ADJUNCT TWO Investigators
- Reynolds J ,
- Battelino T ,
- Liljenquist D ,
- Becker RH ,
- Bergmann K ,
- Lehmann A ,
- Cheng AYY ,
- Stender-Petersen K ,
- Hövelmann U ,
- Carlson AL ,
- Komatsu M ,
- Coutant DE ,
- Stamati A ,
- Karagiannis T ,
- Christoforidis A
- Heinemann L ,
- Baughman R ,
- Akturk HK ,
- Snell-Bergeon JK ,
- Prabhu JN ,
- Seyed Ahmadi S ,
- Westman K ,
- Pivodic A ,
- Hermann JM ,
- Freiberg C ,
- DPV Initiative
- Dicembrini I ,
- Cosentino C ,
- Mannucci E ,
- Abelseth J ,
- Karageorgiou V ,
- Papaioannou TG ,
- Weisman A ,
- Cardinez M ,
- Kramer CK ,
- Asarani NAM ,
- Reynolds AN ,
- Elbalshy M ,
- Jennings P ,
- Burnside MJ ,
- Williman JA ,
- Fridell JA ,
- Stratta RJ ,
- Gruessner AC
- Robertson RP
- Shapiro AM ,
- Pepper AR ,
- Shapiro AMJ
- Walker JT ,
- Saunders DC ,
- Brissova M ,
- Gitelman SE ,
- Bluestone JA
- Hagopian W ,
- Ferry RJ Jr . ,
- Protégé Trial Investigators
- von Scholten BJ ,
- Kreiner FF ,
- Gough SCL ,
- von Herrath M
- Type 1 Diabetes TrialNet Abatacept Study Group
- Hardie DG ,
- Dejgaard TF ,
- Christiansen E ,
- ADJUNCT ONE and ADJUNCT TWO Investigators
- Yunasan E ,
- Peters AL ,
- Palanca A ,
- van Nes F ,
- Ampudia Blasco FJ ,
- Dobbins RL ,
- Greenway FL ,
- Zambrowicz BP ,
- Brodovicz K ,
- Soleymanlou N ,
- Wissinger E ,
- Juhaeri J ,
- Mayer-Davis EJ
- Dhatariya KK ,
- Glaser NS ,
- Umpierrez GE
- Ramphul K ,
- Umpierrez G ,
- Korytkowski M
- Weinstock RS ,
- T1D Exchange Clinic Network
- Eshkoli T ,
- Brandstaetter E ,
- Jotkowitz A
- Dhatariya KK
- Joint British Diabetes Societies for Inpatient Care
- Kilpatrick ES ,
- Butler AE ,
- Ostlundh L ,
- Koufakis T ,
- Agiostratidou G ,
- International Hypoglycaemia Study Group
- van de Ven KC ,
- Heerschap A ,
- van der Graaf M ,
- de Galan BE
- Alsifri S ,
- Aronson R ,
- HAT Investigator Group
- Pettus JH ,
- Shepherd L ,
- Van Houten HK ,
- Ziegenfuss JY ,
- Wermers RA ,
- Schernthaner G ,
- Anderson J ,
- Saunders AL ,
- Snell-Bergeon J ,
- Forlenza GP ,
- Bispham J ,
- Gabbay RA ,
- Pontiroli AE ,
- Tagliabue E
- T1D Exchange Intranasal Glucagon Investigators
- Freckmann G ,
- Ehrmann D ,
- El Laboudi A ,
- Spanudakis E ,
- Anantharaja S ,
- Peleckis AJ ,
- Dalton-Bakes C ,
- van Beers CA ,
- Kleijer SJ ,
- CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group ,
- Piras de Oliveira C ,
- Ribeiro A ,
- Chigutsa F ,
- Malecki MT ,
- Hopkins D ,
- Lawrence I ,
- Mansell P ,
- Stanton-Fay SH ,
- Hamilton K ,
- Chadwick PM ,
- DAFNEplus study group
- Goldsmith K ,
- Yudkin JS ,
- Buntinx F ,
- Mapatano MA ,
- De Clerck M ,
- Truyers C ,
- Chambers D ,
- O’Cathain A
- Klatman EL ,
- Auzanneau M ,
- Tanenbaum ML ,
- Iturralde E ,
- Lipman TH ,
- Bhutta ZA ,
- Pfiester E ,
- Thieffry A ,
- Ballhausen H ,
- Gajewska KA ,
- O’Donnell S
- Wareham NJ ,
- Joosse HJ ,
- Raposo JF ,
- de Courten M
- Hemmingsen B ,
- Abouhassan T ,
- Albanese-O’Neill A ,
- Jacobsen L ,
- Haller MJ ,
- Castorino K ,
- Lovblom LE ,
- Cardinez N ,
- Nguyen KT ,
- Andersen G ,
- Meiffren G ,
- Famulla S ,
- Castellanos LE ,
- Balliro CA ,
- Sherwood JS ,
- Tsoukas MA ,
- Bernier-Twardy S ,
- Martinson LA ,
- Carlsson PO ,
- Schwarcz E ,
- Korsgren O ,
- Oliveira MC ,
- Stracieri AB ,
- Buzzetti R ,
- Mauricio D ,
- McGibbon A ,
- Ingersoll K ,
- Tugwell B ,
- Diabetes Canada Clinical Practice Guidelines Expert Committee
- Fleming GA ,
- McCall AL ,
- Gianchandani R ,
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Type 1 diabetes.
Jessica Lucier ; Priyanka M. Mathias .
Last Update: October 5, 2024 .
- Continuing Education Activity
Type 1 diabetes is a disease involving the immune-mediated destruction of insulin-producing pancreatic β-cells, leading to insulin deficiency. Type 1 diabetes is less common in adults than in younger individuals and should be distinguished from type 2 diabetes, which is more prevalent in older populations. Individuals with type 1 diabetes require lifelong exogenous insulin replacement. Without insulin, patients can develop severe hyperglycemia and, ultimately, diabetic ketoacidosis, which can be life-threatening. Besides insulin therapy, glucose monitoring, disease-specific education, diet, and lifestyle modifications are cornerstones of type 1 diabetes management. Diabetes self-management education and support should include training on blood glucose monitoring, insulin administration, ketone testing when indicated, nutrition education, methods to avoid and treat hypoglycemia, and sick day rules.
This activity for healthcare professionals is designed to enhance learners' proficiency in evaluating and managing type 1 diabetes. Participants gain a broader grasp of the condition's epidemiology, etiology, potential complications, and evidence-based diagnostic and therapeutic strategies. Greater competence enables clinicians to collaborate effectively within an interprofessional team caring for patients with type 1 diabetes, improving outcomes.
- Identify the pathophysiological mechanisms underlying type 1 diabetes, including the role of autoimmune processes and genetic factors, to better inform diagnosis and treatment strategies.
- Implement the latest advancements in diabetes technology to optimize glycemic control.
- Differentiate between type 1 and type 2 diabetes, especially in adults, to guide appropriate treatment strategies.
- Implement effective collaboration and communication among interprofessional team members to improve outcomes and treatment efficacy for patients with type 1 diabetes.
- Introduction
Type 1 diabetes (T1D) is a condition characterized by the immune-mediated destruction of insulin-producing pancreatic β-cells, leading to absolute insulin deficiency. The metabolic, genetic, and immunogenetic characteristics of T1D are heterogeneous, with age-related differences necessitating a personalized approach for each individual. Underlying genetic risk is present in many individuals with the disease. Hence, the American Diabetes Association (ADA) recommends that first- and second-degree relatives of individuals with T1D be screened and offered T1D autoantibody testing. [1]
Individuals with multiple T1D-related autoantibodies eventually develop clinical disease. The loss of insulin secretion can occur gradually or rapidly. Classic symptoms at the onset include polyuria, polydipsia, and unintentional weight loss, but the clinical presentation varies individually. Adults with new-onset T1D usually present with symptoms similar to those seen in children but may have a more gradual progression.
Diabetic ketoacidosis is more prevalent among young patients with new-onset T1D. [2] Disease-modifying therapy has now been approved in the early preclinical stages of T1D to delay the onset of clinical diabetes. [3] Other immune-modifying therapies to delay disease onset in at-risk patients are also being studied.
Successful T1D management requires an interprofessional approach to patient care. Besides insulin replacement therapy, diabetes self-management education, nutrition support, and effectively recognizing and managing coexisting psychological issues are essential for optimizing T1D outcomes. A collaborative, interprofessional approach is recommended, involving many healthcare professionals, including nurses, dietitian educators, pharmacists, community resources, and specialists as needed, such as podiatrists, mental health professionals, social workers, ophthalmologists, and cardiologists. [4]
T1D results from the autoimmune destruction of the β-cells in Langerhans pancreatic islets, ultimately leading to absolute insulin deficiency. [5] This disease manifests in genetically susceptible individuals in whom the autoimmune process is triggered by one or more environmental factors, resulting in immune-mediated β-cell destruction. The loss of β-cell function progresses gradually over months to years, during which time the affected individual remains asymptomatic. Symptomatic hyperglycemia develops when a significant amount of β-cell dysfunction occurs.
Genetic Associations of Type 1 Diabetes
The exact etiology of T1D remains unknown. However, a genetic predisposition is strongly associated with specific human leukocyte antigen (HLA) alleles DR and DQ. HLA genes have been reported to account for approximately 40% of the familial aggregation of T1D. The HLA class II DRB1, -DQA1, -DQB1 genotypes confer the strongest genetic risk factors for T1D. [6] Specifically, HLA DR4-DQ8 and DR3-DQ2 have been reported to be present in about 90% of children with T1D.
The lifetime risk of developing T1D is significantly increased in close relatives of a patient with T1D. However, most cases occur in patients without any family history of T1D or other autoimmune disease. This association is more pronounced in youth-onset than adult-onset T1D. [7] Multiple other genes also contribute to heritability. [8] Screening of family members must be considered, especially first-degree relatives of individuals with T1D, to identify people who may be at risk.
Environmental Risk Factors
Environmental factors are generally believed to trigger autoimmune β-cell destruction in genetically susceptible people. Some studies have found an increased T1D risk related to infection with Coxsackie virus, enteroviruses, cytomegalovirus, rubella virus, influenza B, mumps virus, and more recently, SARS-CoV-2 (COVID-19). [9] [10] [11] Other environmental factors that may increase risk include pregnancy and perinatal conditions, childhood vaccination, and dietary factors such as cow's milk and cereal exposure. Research to better understand the exact role of these environmental agents in the etiology of T1D is ongoing.
Autoimmunity
Besides genetic and environmental factors, several T1D-related autoantibodies target pancreatic β-cell autoantigens, leading to immune-mediated β-cell destruction. Autoantibody targets include antigens in the islet cell cytoplasm (ICA), insulin (IAA), glutamic acid decarboxylase isoform 65 (GAD65), insulinoma antigen 2/islet tyrosine phosphatase 2 (IA-2), and zinc transporter isoform 8 (ZnT8). IAAs are primarily detected in children. [12] GAD65 is the most common autoantibody detected in adults. [13] Testing for autoantibodies to pancreatic β-cell autoantigens is important in confirming the diagnosis and distinguishing T1D from other forms of diabetes, mainly type 2 diabetes (T2D). The greater the number of detectable antibodies and the higher their titers, the greater the risk of developing T1D.
- Epidemiology
T1D is one of the most frequent chronic diseases in children, but the disease can affect any age group. Childhood-onset T1D tends to present with more severe clinical presentations, including symptomatic severe hyperglycemia or diabetic ketoacidosis (DKA). In adults, new-onset T1D may be misdiagnosed as T2D, but youth-onset T1D is more common than adult-onset T1D. Although autoimmune disease tends to be more commonly seen in women, T1D appears to be slightly more common in men. [14]
T1D incidence and prevalence have steadily increased, now representing approximately 5% to 10% of people with diabetes. A systematic review and meta-analysis reported that the worldwide prevalence of T1D was 9.5%, with an incidence of 15 per 100,000 people. [15] Worldwide, T1D's geographic incidence varies considerably. The highest reported incidences are in Finland and other Northern European nations, with rates approximately 400 times greater than those seen in China and Venezuela, where incidence is reportedly the lowest.
- Pathophysiology
The natural history and development of T1D in genetically susceptible individuals occur in 3 stages. Stage 1, the preclinical stage, is characterized by the onset of autoimmune β-cell destruction and insulitis caused by immune-mediated destruction. This stage is asymptomatic and characterized by normal fasting glucose, normal glucose tolerance, and the presence of at least 2 pancreatic autoantibodies. In Stage 2, a significant amount of β-cell dysfunction has already occurred, leading to dysglycemia. The diagnostic criteria include the presence of pancreatic autoantibodies with impaired fasting glucose (fasting glucose 100-125 mg/dL), impaired glucose tolerance (2-hour post-75 g glucose load glucose 140-199 mg/dL), or a glycated hemoglobin (HbA1c) level of 5.7% to 6.4%. Individuals remain asymptomatic.
Stage 3 is characterized by the clinical onset of disease where individuals present with symptomatic hyperglycemia. The diagnostic criteria include diabetes, defined by hyperglycemia (random glucose ≥200 mg/dL) with clinical symptoms, fasting glucose of at least 126 mg/dL, blood glucose level of at least 200 mg/dL 2 hours after ingesting 75 g of glucose during an oral glucose tolerance test, or HbA1c greater than or equal to 6.5%. T1D classically presents with symptomatic hyperglycemia, especially in children. Individuals with classic new-onset T1D usually present with symptoms of polydipsia, polyuria, polyphagia, unintentional weight loss, fatigue, and weakness. Life-threatening DKA can develop if T1D is not evaluated and treated promptly.
DKA is characterized by hyperglycemia, ketonuria, and electrolyte disturbances that lead to metabolic acidosis. Besides polyuria, polydipsia, and unintentional weight loss, patients in DKA may present with fruity-smelling breath, lethargy, and, in severe cases, even coma. Early detection and initiation of treatment, including intravenous fluids, insulin, potassium, and careful monitoring, is important. Most patients require admission to an intensive care unit for management and monitoring. The incidence of DKA in children ranges anywhere between 15% and 70%. [16]
The onset of symptoms in adults is more variable than in younger patients, and DKA is less common. Patients are often misdiagnosed with T2D and later found to be insulin-dependent. T1D can be difficult to distinguish from T2D. Screening for T1D antibodies and family history are important in confirming the diagnosis.
GAD65 should be the initial antibody tested when T1D in adults is suspected. IA2 or ZNT8 should be measured if GAD65 testing is negative or unavailable. C-peptide levels may also be used to determine β-cell function and the degree of insulin dependency when the diagnosis is still unclear. In patients with T1D, fasting insulin and C-peptide levels are inappropriately low when the concomitant plasma glucose concentration is elevated. By contrast, elevated fasting insulin and C-peptide levels suggest T2DM.
- History and Physical
After the initial diagnosis and medical stabilization, successful management of T1D involves improving glycemic control, preventing long-term complications and sequelae of hyperglycemia, and T1D education while maintaining normal growth and development in children and improving quality of life. Initial diabetes education provided by an interprofessional care team is essential for the patient and family to acquire the knowledge needed to manage this chronic disease. Clinicians need to reinforce that multiple factors impact glycemic control and involve the patient or family in a comprehensive treatment plan that emphasizes a healthy lifestyle, which can improve disease outcomes.
At the initial outpatient visit, obtaining a complete medical, psychosocial, and family history, including pregnancy and contraception history, is essential. History of prior diabetes education, monitoring of blood glucose and ketones, administration of insulin, and recognition and treatment of hypoglycemia should be obtained. Particular attention should be paid to the date of diagnosis, prior treatment received, knowledge of sick day rules, and history of acute complications (severe hypoglycemia or DKA) and chronic complications (eg, skin disorders, dental problems, diabetic retinopathy, diabetic neuropathy, kidney disease, cardiovascular disease, peripheral arterial disease, stroke, foot ulcers, and foot amputations).
Since people with T1D have an increased risk of developing other autoimmune disorders, including autoimmune thyroid pathology and celiac disease, the clinician should also screen for these conditions during clinical evaluation. Several measures are available for psychosocial screening, such as the Patient Health Questionnaire (PHQ-2/PHQ-9) for Depression and Generalized Anxiety Disorder (GAD-7). Diabetes distress and social determinants of health should be assessed. Eating disorders are common in individuals with type 1 diabetes, particularly young women. Thus, patients should be examined for this problem. Early cognitive decline is also common in adults. Therefore, cognitive testing should be considered when impairment is suspected.
A complete physical examination is also performed. A diabetes foot examination must be included to detect early peripheral neuropathy signs, foot deformities, pre-ulcerative lesions, ulcerations, calluses, and onychomycosis. Testing vibratory and protective sensations is also essential. Abnormal testing with a 10-g monofilament exam suggests an increased risk of ulceration. The skin should be examined, especially at insulin injection or infusion sites. If lipodystrophy is evident, patients should be educated on the importance of varying insulin injections or infusion sites.
Patients with T1D can present with classic symptoms of new-onset diabetes, such as polyuria, polydipsia, lethargy, and weight loss. These individuals may also present more acutely with DKA. Other clinical manifestations include acute visual disturbances, perineal candidiasis, or, in some adults, an initial misdiagnosis of T2D before correctly identifying T1D.
Diabetes may be diagnosed using plasma glucose criteria, such as fasting plasma glucose or postprandial glucose during a 75-g oral glucose tolerance test (OGTT), or based on HbA1c levels. Diagnostic criteria for diabetes include the following:
- Fasting plasma glucose of at least 126 mg/dL on more than 1 occasion
- Random plasma glucose of at least 200 mg/dL with classic symptoms of hyperglycemia
- Plasma glucose of at least 200 mg/dL measured 2 hours after a 75-g OGTT
- HbA1C level of at least 6.5% [17]
In the absence of unequivocal hyperglycemia, the diagnosis is confirmed based on 2 abnormal test results. Once the diagnosis of diabetes is confirmed, distinguishing between T1D and other forms of diabetes, mainly T2D, is critical. These conditions may be differentiated based on clinical presentation and laboratory studies, including testing for T1D pancreatic autoantibodies and stimulated C-peptide levels, with the latter measuring pancreatic β-cell function. T1D pancreatic antibodies include ICA, IAA, GAD65, IA-2, and ZnT8. Most patients with T1D have 1 or more positive T1D antibodies at the time of diagnosis.
Evaluating glycemic control by checking HbA1c levels is recommended every 3 months during each follow-up visit. Other laboratory tests that should be conducted, if not performed within the past year, include a lipid profile, serum creatinine, spot urinary albumin-to-creatinine ratio, liver function tests, thyroid-stimulating hormone, complete blood count with platelets, and serum potassium—especially if the patient is also taking an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or diuretic. Since people with T1D are at an increased risk of developing other autoimmune diseases, such as autoimmune thyroid conditions, celiac disease, primary adrenal insufficiency, and rheumatoid arthritis, screening for autoimmune disorders should be considered when clinically appropriate. [18]
- Treatment / Management
Individuals with T1D require lifelong insulin replacement, regular blood glucose monitoring, and adjustments in diet and lifestyle to achieve optimal glycemic control. Glycemic targets should be personalized based on each patient's health status and coexisting conditions, guiding treatment strategies. According to the ADA, optimal HbA1c levels should be reached to minimize the risk of microvascular and macrovascular complications while preventing hypoglycemia. For most patients, an HbA1c goal of less than or equal to 7% is recommended, though older individuals or those with multiple health issues may benefit from more relaxed HbA1c targets.
Insulin Replacement
The goal in T1D management is physiologic replacement of insulin, either by administering multiple daily insulin injections (MDI) or continuous subcutaneous insulin infusion via an insulin pump. MDI or "basal-bolus" insulin therapy includes basal long-acting insulin (administered once or twice a day) and prandial or mealtime insulin using short-acting or rapid-acting insulin multiple times a day before meals. Continuous subcutaneous insulin infusion includes administering a rapid- or short-acting insulin continuously via an insulin pump with additional boluses initiated on the pump for prandial coverage at mealtimes.
Multiple daily injections
Various insulin types may be used for insulin injection therapy. [19] The choice of basal and prandial insulin for MDI usually depends on patient preference, insurance coverage, availability, and cost. Long-acting insulin is preferred for basal insulin injection therapy, often given once a day (U-100 and U-300 glargine, degludec) or 1 to 2 times daily (detemir and U-100 glargine).
Glargine does not have a pronounced peak and lasts approximately 20 to 24 hours. U-300 glargine lasts more than 24 hours, and degludec has a longer duration of action, up to 42 hours. Intermediate insulin, eg, neutral protamine Hagedorn or neutral protamine lispro, is the least expensive basal insulin but is more likely to cause hypoglycemia. Intermediate insulin's action onset is 1 to 2 hours. Peak action is at 2 to 8 hours, and duration is 12 to 24 hours. This insulin form is usually given before breakfast and bedtime. When MDI is used, the patient should inject rapid-acting insulin with each meal for hyperglycemia correction and a daily long-acting basal insulin.
For prandial or mealtime coverage, options include rapid-acting insulin, ultra-rapid-acting insulin, and short-acting regular insulin. This type of insulin is usually administered within 10 to 15 minutes before meals. Rapid-acting insulin (lispro, aspart, glulisine) generally has an onset of 12 to 30 minutes, peaks in 1 to 3 hours, and has a duration of action of 3 to 6 hours. Ultra-rapid-acting lispro or aspart has a slightly quicker onset and somewhat shorter duration. Short-acting insulin (regular insulin) has an onset of 30 minutes to 1 hour, peaks in 2 to 4 hours, and has a duration of 5 to 8 hours. MDI regimens must be titrated to target a blood glucose range of, usually, 79 to 180 mg/dL while minimizing the risk of hypoglycemia (<70 mg/dL) and hyperglycemia (>180 mg/dL).
Continuous subcutaneous insulin infusion
Continuous subcutaneous insulin infusion (CSII) or insulin pump therapy administers a continuous infusion of insulin, usually a rapid-acting insulin, to replace the basal insulin requirement. Besides basal insulin, mealtime insulin boluses are administered via the pump for prandial coverage. The insulin pump consists of the pump itself, a reservoir or cannula for holding insulin, insulin tubing, and a cannula or needle inserted subcutaneously. Advances in diabetes technology have led to newer insulin pumps that connect to continuous glucose monitors (CGMs) and enable automated insulin delivery to target optimal glycemic control.
Several types of insulin pumps are available. Sensor-augmented insulin pumps function independently from CGMs. Predictive low-glucose suspend insulin pumps are programmed to halt insulin delivery when blood glucose drops to a prespecified low threshold. Automated insulin delivery or hybrid closed-loop systems integrate an insulin pump with a connected CGM to automatically adjust insulin delivery based on CGM values and maintain blood glucose within the target range. Do-it-yourself automated insulin delivery systems are also known as "looping."
Blood Glucose Monitoring
Monitoring blood glucose is an integral part of T1D management. Monitoring can be accomplished through capillary blood sampling with a glucose meter or CGM systems that provide real-time glucose data via a sensor and reader or smartphone app.
Glucose meter
Patients using a blood glucose meter should check their blood sugar levels at least 4 times a day, including before meals and at bedtime. Numerous blood glucose meters are available on the market, and the choice of meter often depends on cost or insurance coverage.
Continuous glucose monitors
CGMs have now become the standard of care in diabetes management. These devices are especially important in T1D management, as patients have an increased risk of frequent and severe hypoglycemia and hypoglycemia unawareness. Real-time CGM devices measure glucose every few minutes and automatically transmit glucose data to a receiver or phone application device. The device can also alarm when hypoglycemia or hyperglycemia triggers are met. CGMs collect real-time blood glucose data and trends, allowing patients to anticipate blood glucose levels for the next 30 to 60 minutes and adjust treatment decisions accordingly. Intermittent or flash-type glucose monitoring devices are also available. These devices require patients to periodically scan their sensors with a reader or phone application to obtain blood glucose readings.
Diabetes Education and Self-Management
Optimal T1D management includes intensive education on diet, lifestyle, insulin management, and blood glucose monitoring. Treatment regimens and goals are often complex, requiring patients to receive ongoing education and self-management support to achieve these goals. Patients must understand the interaction between insulin, diet, physical activity, and daily activities on their blood glucose levels. They also need to appreciate the importance of adhering to their insulin regimen and monitoring blood glucose. Education should also cover ketone monitoring, sick day rules, detection and early treatment of hypoglycemia, and screening for diabetes complications. When appropriate, the patient’s family and caregivers should also be educated to provide adequate support.
Nutrition education, including carbohydrate estimation and counting, is important in diabetes management. Carbohydrate counting is often essential for patients to achieve optimal mealtime blood glucose targets and to accurately estimate prandial insulin needs. Carbohydrate estimation helps reduce the risk of hypoglycemia. For example, patients may have an increased risk of postprandial hypoglycemia if they consume a low-carbohydrate meal but administer the full amount of mealtime insulin without adjusting for the actual carbohydrate content. Patients should consult with a dietitian when possible to learn carbohydrate counting and be instructed to use an insulin-to-carbohydrate ratio (grams of carbohydrate covered by 1 unit of insulin) for mealtime dosing. If carbohydrate counting is not feasible, maintaining a carbohydrate-consistent diet may be the best alternative. Besides dietary education, incorporating physical activity is important for people living with T1D. Physical activity is encouraged for its beneficial effects on insulin sensitivity and overall health, but it requires careful management to avoid glucose fluctuations.
Hypoglycemia
Hypoglycemia is the most frequent adverse effect of insulin therapy. Educating people living with T1D and their partners about the signs and symptoms of hypoglycemia is crucial. Symptoms include sweating, rapid heartbeat, lightheadedness, confusion, hunger, visual changes, and tremors. Symptoms of hypoglycemia typically occur when blood glucose levels drop below 70 mg/dL, known as level 1 hypoglycemia. Level 2 hypoglycemia occurs when blood glucose levels fall below 54 mg/dL and is usually associated with cognitive impairment and altered consciousness. Level 3 hypoglycemia involves a hypoglycemic episode that requires assistance from another person for resuscitation.
Recurrent hypoglycemic episodes, particularly in individuals with a longer duration of T1D, can lead to hypoglycemia unawareness. This condition occurs when individuals experience hypoglycemia symptoms at progressively lower glucose thresholds, likely due to reduced sympathoadrenal responses or autonomic failure. Hypoglycemia not only impacts the quality of life in people living with T1D but also increases the risk of cardiovascular events and mortality. In older adults or frail individuals, hypoglycemia can heighten the risk of falls, cognitive impairment, dementia, and fractures.
Patients must be educated about the signs and symptoms of hypoglycemia, the blood glucose thresholds for treatment, and the use of glucagon for severe hypoglycemia unresponsive to conventional treatments. Individuals at risk for recurrent and severe hypoglycemia benefit from using a CGM, which can alert them to treat hypoglycemia promptly. Hypoglycemia treatment generally involves consuming 15 to 20 grams of glucose orally if blood glucose levels fall below 70 mg/dL. Blood glucose should be rechecked 15 minutes later, with additional carbohydrates administered if necessary. Once blood glucose levels have normalized, a snack should be provided to prevent recurrence. Glucagon should be prescribed for emergency use in cases of severe hypoglycemia when the individual cannot consume carbohydrates orally.
Follow-up and Ongoing Care
Following the initial evaluation and diabetes visit, the diabetes care team should continue to provide T1D education and support while assessing the patient's insulin regimen and blood glucose control. Regular visits with the endocrinologist, diabetes educator, nurse practitioner, dietitian, and, if necessary, mental health professionals ensure comprehensive education and care aimed at maintaining optimal glycemic control. At each follow-up visit, key aspects of glycemic control, insulin management, the interaction between diet and exercise, and the treatment regimen should be reinforced. The patient's blood glucose data, whether from a glucose monitoring device or CGM, should be reviewed in detail with the patient.
For patients using an insulin pump or automated insulin delivery device, data from the device should be downloaded and discussed when appropriate. Adjustments to the patient's treatment regimen should be reviewed and reinforced as needed. Additionally, the clinician should screen for diabetes complications and comorbidities at follow-up visits and arrange referrals to specialists if necessary. Effective ongoing T1D management should also involve evaluating overall health status and engaging in shared decision-making to set treatment goals.
- Differential Diagnosis
T1D must be distinguished from similar conditions based on the patient's clinical presentation, history, and laboratory studies. These conditions include type 2 diabetes, monogenic diabetes, diseases affecting exocrine pancreas function (cystic fibrosis-related diabetes, chronic pancreatitis0, posttransplantation diabetes mellitus, steroid-induced diabetes, and psychogenic polydipsia.
T1D is a challenging condition to manage and can lead to complications that can shorten life expectancy. However, morbidity and mortality associated with the disease have improved with advances in insulin therapy and diabetes technology, improvement in glycemic control, and control of metabolic risk factors such as hypertension and hyperlipidemia. Timely screening for microvascular and macrovascular complications and strict glycemic control at disease onset have reduced rates of serious diabetes-related complications. Mortality rates have declined, though people with T1D have 2- to 5-fold higher mortality than those without diabetes. This issue is discussed further in other sections. [20]
- Complications
Complications secondary to T1D can be classified into acute and chronic conditions. Acute complications include hypoglycemia and DKA. The most serious chronic complications include nephropathy, peripheral and autonomic neuropathy, retinopathy, heart disease (including coronary artery disease, heart failure, and cardiomyopathy), peripheral arterial disease, cerebrovascular disease (including stroke and transient ischemic attack), and diabetic foot infections.
- Deterrence and Patient Education
Successful T1D management requires an interprofessional approach to patient care. Patient medication compliance and follow-up with specialists and educators are critical factors in preventing complications. At every patient encounter, the pharmacist, nurse, and clinician should emphasize the importance of blood glucose control, screening for long-term complications, and diabetes management goals. The patient should be encouraged to modify their lifestyle to reduce the risk of complications. All individuals with diabetes should also be made aware of the signs and symptoms of hypoglycemia and ways to prevent and treat this condition. Patients should be educated about available resources and the benefits of joining diabetes support groups when needed.
- Enhancing Healthcare Team Outcomes
Self-management of type 1 diabetes (T1D) involves MDI or insulin administration via an insulin pump, along with glucose monitoring and careful attention to diet and physical activity. This daily regimen can be burdensome and, in some individuals, eventually lead to diabetes distress or burnout. While technological advancements have enabled better glycemic control, these innovations often come with high costs, complexity, and the need for extensive education and training. Additionally, many individuals with diabetes experience anxiety about hypoglycemia, hyperglycemia, and potential complications, which can lead to mental health issues such as depression, anxiety, and eating disorders.
Addressing the comprehensive medical, educational, psychological, and social challenges faced by people living with T1D requires an interprofessional team approach. This team typically includes primary care clinicians, endocrinologists, diabetes nurse educators, pharmacists, dietitians, mental health professionals, social workers, podiatrists, and community resource representatives. By adopting individualized treatment plans, which can lessen the burden of care and further enhance outcomes, the interprofessional care model is positioned to achieve the best possible patient results.
All members of the interprofessional team need to synchronize their efforts and maintain open communication to ensure that all parties involved in patient care, including the patients themselves, have access to accurate and updated information. Nurses are pivotal in coordinating activities among various healthcare professionals and contribute significantly to patient evaluation, education, and monitoring. Pharmacists, working closely with diabetes educators, should ensure proper insulin dosing and participate in patient education and medication reconciliation. These examples of interprofessional collaboration are key to driving improved patient outcomes.
- Review Questions
- Access free multiple choice questions on this topic.
- Click here for a simplified version.
- Comment on this article.
Disclosure: Jessica Lucier declares no relevant financial relationships with ineligible companies.
Disclosure: Priyanka Mathias declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Lucier J, Mathias PM. Type 1 Diabetes. [Updated 2024 Oct 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Recommendations for Screening and Monitoring the Stages of Type 1 Diabetes in the Immune Therapy Era. [Int J Gen Med. 2024] Recommendations for Screening and Monitoring the Stages of Type 1 Diabetes in the Immune Therapy Era. Moore DJ, Leibel NI, Polonsky W, Rodriguez H. Int J Gen Med. 2024; 17:3003-3014. Epub 2024 Jul 9.
- Early Intervention by Family Physicians to Delay Type 1 Diabetes. [J Fam Pract. 2023] Early Intervention by Family Physicians to Delay Type 1 Diabetes. Edelman S. J Fam Pract. 2023 Jul; 72(6 Suppl):S19-S24.
- Age of Diagnosis Does Not Alter the Presentation or Progression of Robustly Defined Adult-Onset Type 1 Diabetes. [Diabetes Care. 2023] Age of Diagnosis Does Not Alter the Presentation or Progression of Robustly Defined Adult-Onset Type 1 Diabetes. Thomas NJ, Hill AV, Dayan CM, Oram RA, McDonald TJ, Shields BM, Jones AG, StartRight Study Group. Diabetes Care. 2023 Jun 1; 46(6):1156-1163.
- Review Type 1 diabetes: A predictable disease. [World J Diabetes. 2015] Review Type 1 diabetes: A predictable disease. Simmons KM, Michels AW. World J Diabetes. 2015 Apr 15; 6(3):380-90.
- Review Anti-Islet Autoantibodies in Type 1 Diabetes. [Int J Mol Sci. 2023] Review Anti-Islet Autoantibodies in Type 1 Diabetes. Kawasaki E. Int J Mol Sci. 2023 Jun 11; 24(12). Epub 2023 Jun 11.
Recent Activity
- Type 1 Diabetes - StatPearls Type 1 Diabetes - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Open access
- Published: 01 April 2021
Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era
- Ammira Al-Shabeeb Akil ORCID: orcid.org/0000-0001-5381-070X 1 ,
- Esraa Yassin 1 ,
- Aljazi Al-Maraghi 1 ,
- Elbay Aliyev 1 ,
- Khulod Al-Malki 1 &
- Khalid A. Fakhro 1 , 2 , 3
Journal of Translational Medicine volume 19 , Article number: 137 ( 2021 ) Cite this article
45k Accesses
62 Citations
2 Altmetric
Metrics details
Type 1 diabetes affects millions of people globally and requires careful management to avoid serious long-term complications, including heart and kidney disease, stroke, and loss of sight. The type 1 diabetes patient cohort is highly heterogeneous, with individuals presenting with disease at different stages and severities, arising from distinct etiologies, and overlaying varied genetic backgrounds. At present, the “one-size-fits-all” treatment for type 1 diabetes is exogenic insulin substitution therapy, but this approach fails to achieve optimal blood glucose control in many individuals. With advances in our understanding of early-stage diabetes development, diabetes stratification, and the role of genetics, type 1 diabetes is a promising candidate for a personalized medicine approach, which aims to apply “the right therapy at the right time, to the right patient”. In the case of type 1 diabetes, great efforts are now being focused on risk stratification for diabetes development to enable pre-clinical detection, and the application of treatments such as gene therapy, to prevent pancreatic destruction in a sub-set of patients. Alongside this, breakthroughs in stem cell therapies hold great promise for the regeneration of pancreatic tissues in some individuals. Here we review the recent initiatives in the field of personalized medicine for type 1 diabetes, including the latest discoveries in stem cell and gene therapy for the disease, and current obstacles that must be overcome before the dream of personalized medicine for all type 1 diabetes patients can be realized.
Introduction
Type 1 Diabetes (T1D) is a potentially life-threatening multifactorial autoimmune disorder characterized by T-cell-mediated destruction of pancreatic β cells, resulting in a deficiency of insulin synthesis and secretion [ 1 ]. The incidence of T1D has been rising globally since the 1950s, with an average annual increase of 3–4% over the past three decades [ 2 ]. In particular, the incidence of childhood T1D is increasing, most rapidly in populations that previously had low incidence [ 3 , 4 , 5 ], and varying by ethnicity and race [ 4 ].
This worrying growth in T1D incidence has driven concerted research efforts to better understand the underlying risk factors, etiology, and pathology of the disease.
T1D has a largely heritable element, supported by a twin concordance rate of up to 70% [ 6 ] and of 8–10% sibling risk [ 7 ]. The bulk of risk is explained by difference at a several but strongly associated loci involving the HLA region “HLA class II, DQ and DR loci and HLA class I region” on chromosome 6p21 that account for ~ 50% of familial T1D [ 8 , 9 ]. Genome‐wide association (GWAS) and candidate gene association studies have produced an abundance body of evidence and provided convincing support about other genes and loci external to the HLA region that protect or confer the risk for T1D [ 8 , 10 ]. Single nucleotide polymorphisms (SNPs) comprising insulin gene ( INS ) presents ~ 10% of genetic predisposition of T1D [ 8 , 11 ], cytotoxic T-lymphocyte–associated antigen ( CTLA )-4 gene [ 12 ], protein tyrosine phosphatase non-receptor type 22 ( PTPN22) [ 8 , 13 ], nterferon induced with helicase C domain 1 ( IFIH1 ) genes [ 14 ] and Interleukin-2 receptor alpha chain ( IL2RA ) [ 11 ]. This great genetic heritability generates the capacity for effective diagnostic discrimination if the most of genetic risk for T1D can be allocated [ 15 , 16 ].
Prospective birth cohorts studies have facilitated the identification of potential triggers of islet autoimmunity (IA) and the natural history of progression to T1D [ 17 , 18 , 19 , 20 ]. Candidate triggers such as infections [ 21 ], early life diet [ 22 ], vitamin D levels [ 23 ], gut microbiota composition [ 24 ], vaccinations [ 25 ], pollutants and toxins [ 26 ], and geographic variation [ 27 ] when combine with genetic susceptibility [ 28 ] and specific epigenetic modifications [ 29 , 30 , 31 ], the perfect storm occurs and autoimmune destruction of pancreatic β cells is initiated (Fig. 1 ). These triggers required to be logged prospectively in well-designed studies instead of recollected retrospectively at the time of T1D diagnosis, couple of years later.
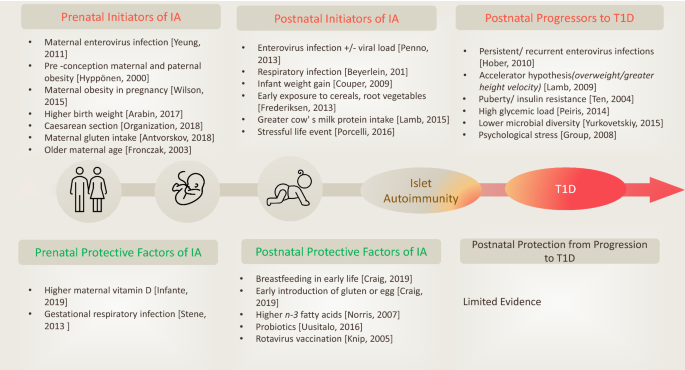
Environmental factors associated with initiation of, or protection from islet autoimmunity (IA) and progression to T1D. Adopted with permission from (Craig et al. 2019)
The plethora of factors that can lead to development and expression of T1D underpin the clinical heterogeneity of the disease. The gene polymorphisms and environmental triggers combinations that impact the risk of T1D and lead to the disease development are tremendously high [ 32 ]. Until now, this heterogeneity has not been taken into account and almost all T1D patients are treated with the standard approach of regular blood glucose monitoring combined with exogenous insulin replacement. However, the rising social and healthcare costs globally associated with T1D and its complications are providing the impetus for prioritizing more tailored approaches [ 33 , 34 , 35 ]. There is now increasing recognition of the opportunity to identify specific patient subgroups at different stages or with different driving factors of their early disease and prevent or even reverse their emerging T1D: this is the concept of personalized medicine. Personalized medicine is characterized by the mantra of "offering the right therapy at the right time for the right affected individual"; as an idea it is not new, but only recently has scientific and clinical research provided us with the necessary information and the means with which to apply it to novel treatment strategies for T1D.
In this review, we bring together the latest knowledge of the factors underpinning T1D heterogeneity in distinct patient groups and how these differences are being used to design personalized medicine approaches to diagnose, prevent, and hopefully treat the disease. We will discuss recent advances in gene therapy and stem cell-based treatments for specific groups of T1D patients, and will highlight key obstacles that must be overcome if further progress towards the goal of personalized medicine for all T1D patients is to be achieved.
Personalized diagnosis of T1D
Although all patients with overt T1D exhibit pancreatic destruction and consequent dysregulation of blood glucose levels, not all cases of the disease are driven by the same factors or along the same timeline. Many patients experience a sometimes prolonged clinically silent phase in which it might have been possible to intervene and prevent or even reverse the course of disease. This knowledge has led to development of a staging classification system for T1D. Even once T1D is clinically evident, we are now beginning to appreciate that not all cases are the same, and that particular sub-types of the disease would benefit from distinct treatment strategies. We discuss both of these important advances within the field below.
Staging classification system for T1D
By dissecting population- and individual-level risk factors for developing T1D, we now know that the disorder exists across developmental spectrum that can be categorized into distinct stages, and the likelihood of an individual developing clinically symptomatic status can be foreseen with considerable accuracy.
All cases are proposed to start with a period of "incubation" where exposure to defined and undefined driving factors creates the conditions for β-cell autoimmunity to emerge. When the process of ß-cell autoimmunity begins, the development towards clinical T1D can be classified into three distinct main stages: (I) asymptomatic ß-cell autoimmunity, defined by the presence of ≥ 2 types of autoantibodies such as GAD65 (GADA), zinc transporter 8 (ZnT8A), insulin (IAA), islet cell antibodies (ICA), insulinoma-associated proteins (IA-2A and IA-2β), with normoglycemia; (II) asymptomatic ß-cell autoimmunity, characterized by the presence of ≥ 2 types of autoantibodies but with dysglycemia, indicating functional damage to ß-cells; and (III) symptomatic T1D recognized by the symptoms of dysglycemia including polyuria or diabetic ketoacidosis (DKA) (Fig. 2 ). The sequence of events from emerging autoimmunity to dysglycemia and then to overt diabetes occurs along this predictable course, but the length of each stage may vary broadly between different individuals [ 36 , 37 , 38 ].
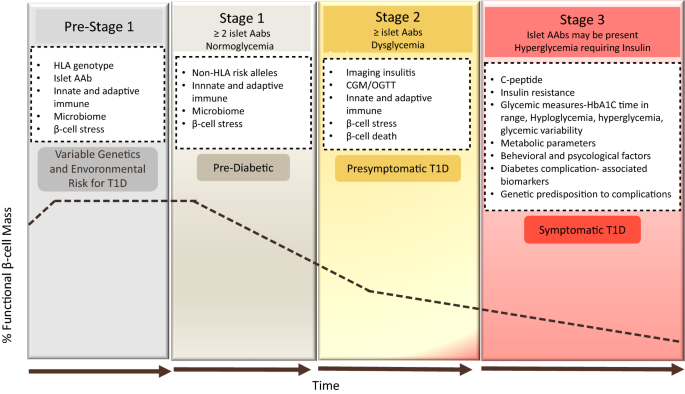
adapted from the same publication on addition to [ 36 ]© 2015 The American Diabetes Association
Development and staging of type 1 diabetes. T1D is characterized by a gradual loss of β-cell function (black dashed-dotted line) over time. As the disease progresses, beta cell function falls below the threshold required to maintain glucose control creating a requirement for insulin replacement therapy. Genetic and environmental risk are both included in the disease etiology. In stage 1, β-cell autoantibodies are persistent, but normoglycemia remains and there are no clinical symptoms. Throughout stage 2, the number of β-cell autoantibodies may induce dysglycemia but still without any diabetes symptoms. In stage 3, β-cell autoantibodies are predominant and clear symptoms of diabetes have emerged. In the white boxes are categories of biomarkers which could be leveraged to refine the staging paradigm, improve prognostic predictions, or subset individuals within a given stage of disease [ 38 ]. The specifics of these biomarkers are discussed in the text related to the relevant stage. The staging of T1D pathogenesis was proposed by Insel et al. [ 36 ] and the figure explanation was
There are several valuable clinical outcomes for children monitored across prospective longitudinal natural history studies such as. Notably, those children have better metabolic markers at and soon after the clinical diagnosis stage, making the disease management relatively easier, reduce hypoglycemic incidents and delay the progress of the associated long-term complications. Rigorous diabetes management commenced afterward the diagnosis of symptomatic T1D increases the chance of a honeymoon phase [ 39 ], assists patients to preserve greater C-peptide ranges [ 40 , 41 ], and reduce mortality rate [ 42 ], indicating that patients who are treated earlier will have improved long-term outcomes. In addition, genetically at risk children of DAISY (Diabetes Autoimmunity Study in the Young) cohort had lower HbA 1C levels maintained within the normal range, a figure much lower than the average HbA 1C levels of T1D children in the community [ 43 , 44 ]. Also, only 3% of the DAISY children were hospitalized at T1D diagnosis compared to 44% of matched children in the community [ 44 ]. The DKA levels was detected in around 30% of the participants of the SEARCH for diabetes in youth study [ 45 ], while the same marker observed in lower prevalence in children screened positive for islet autoantibodies followed by German BABYDIAB and Munich family study [ 46 ].
Children followed by Diabetes Prediction in Skåne (DiPiS) study experienced decreased HbA 1C up to 24 months after the diagnosis against similar daily insulin dose requirements [ 47 ].
The predictable progression of T1D from early stages of autoimmunity to dysglycemia ahead of the symptomatic clinical disease could ease the design of reliable clinical trials using intermediate endpoint that require ~ 50% smaller sample size that those using T1D as the endpoint. In TrialNet natural history study, diabetes- related autoantibodies were analyzed in relatives of T1D patients in respect to elevated HbA 1C, decreased C-peptide following oral glucose tolerance test (OGTT) value as intermediate markers of T1D progression [ 48 ]. Also, the TrialNet CTLA4-Ig (abatacept) ongoing trial designed to test whether intervention with Abatacept could prevent or delay the development of abnormal glucose tolerance (AGT) in at-risk relatives of T1D patients [ 49 ]. Combined predictive risk score for an improved prediction of disease progression by incorporating fixed and variable factors (genetic, immunologic and metabolic markers) in newborn screening to prevent DKA and to enhance personalized risk predication for better T1D prevention trial selection [ 50 , 51 ]. The crucial benefit of utilizing this staging system is to aid in development of innovative, stage-specific diagnostic and predictive biomarkers, support the design of clinical trials that utilizing the available data on risk profiles and individuals’ pre-symptomatic classification to design therapies specifically targeted to each phase of disease and ultimately, practice of personalized medicine approaches to avert symptomatic T1D. Future research will be needed to identify the main drivers of the transitions between stages in order to identify novel therapeutic targets to prevent the emergence of T1D in high-risk populations.
Diagnostic sub-groups within symptomatic T1D
Diagnosis of T1D has historically been made on the basis of detecting blood glucose dysregulation; however, this has led to patients with diverse underlying pathologies being grouped, and treated, together. Evidence of β-cell destruction via the presence of anti-islet-autoantibodies (which may recognize insulin, Glutamic Acid Decarboxylase 65(GAD65), zinc transporter isoform 8 (ZnT8), or islet cell antigen (ICA512) and the age at which initial autoantibodies were detected are important factors that characterize the “classical” etiological subtype of T1D. However, less frequently, hypoglycemia might be caused by loss of function or de novo mutation in a sporadic gene, giving rise to monogenic diabetes, which represents 3% of all diabetes cases in children and adults [ 52 ]. The heterozygous activation of genes encoding the ATP-sensitive potassium-channel subunit Kir6.2 reported to cause permanent neonatal diabetes in addition to some neurological abnormalities in some affected individuals. Distinguishing monogenic diabetes from T1D is crucial for accurate diagnosis, applying the correct treatment “such as sulfonylureas in Kir6.2 mutation”, and in the future, stratifying these patients into a group most likely to benefit from gene therapy targeting the mutation.
The aim of increasing correct diagnosis of classical versus monogenic T1D has been assisted by the introduction of the genomic risk score (GRS), which assesses an individual’s risk of T1D based on their possession of a collection of multiple (10–40) T1D risk variants [ 53 , 54 ]. The GRS also effectively identifies those individuals with early-onset or pre-clinical T1D who show more autoimmunity and fewer syndromic features in comparison with those of monogenic diabetes [ 55 ]. The sensitivity and specificity of the T1D-GRS exceeds 80% [ 55 ], but this figure might reasonably expect to be increased when the GRS is combined with the available clinical data and autoantibody results. Accordingly, incorporating the T1D-GRS into strategies aimed at intervening in the pre-symptomatic T1D stages noted above (Fig. 1 , [ 31 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 ]) is likely to prove productive in the development of personalized diabetes-preventative therapies targeting either mutational correction or prevention of overt autoimmunity.
Somewhat surprisingly, T1D and type 2 diabetes (T2D) are often distinguished based on whether the person exhibiting blood glucose dysregulation is young and a healthy weight (T1D-typical), or instead an older adult with obesity (T2D-typical). However, these two manifestations have different causes and medication requirements [ 80 ]. Research in 2017 found that approximately 40% of people who developed T1D after the age of 30 were initially diagnosed and treated for T2D [ 81 ]. Given the potentially life-threatening nature of insulin-deficiency status [ 81 , 82 ], these findings call for increased use of autoantibody testing to discriminate T1D and T2D, and widespread recognition of the fact that clinical features alone cannot reliably distinguish these two conditions.
Current advances in affordable high-throughput genomic and molecular deep phenotyping technologies have pushed the rise of “next-generation epidemiology” with a more systematic focus than before. In particular, deep phenotyping can be described as the precise and broad analysis of phenotypic data to aid in identifying disease biomarkers that assist the prediction, prevention and disease monitoring [ 83 ]. Recently, an integrative multi-omics approaches were used on the Environmental Determinants of Diabetes in the Young (TEDDY) children, a prospective longitudinal birth cohort created to study T1D by following children with high genetic risk [ 84 ]. The analysis identified a multi-omics signature that able to predict the IA before seroconversion in one year, in addition, defects in lipid metabolism, problems with nutrient absorption, reactive oxygen species (ROS) detected prior to the IA progression.
In conclusion, identification of high risk for T1D genetic groups in the pre-symptomatic stages, coupled with the use of autoantibody testing, GRS and molecular deep phenotyping through utilizing the advanced integrative data analysis, could support the development of approaches for early diagnosis and treatment of T1D in both symptomatic and pre-symptomatic patients. This strategy could form the mainstay of accurate “personalized diagnoses” moving forward. Understanding the genetic etiology and specific pathophysiology of these distinct patient groups within the T1D family will be necessary for the rationale design and application of personalized therapies in the future.
Personalized treatment of T1D
Progress in recognition of the need for personalized diagnosis in T1D has been accompanied by intense research efforts towards personalized therapies. Before the discovery of insulin in 1921, it was remarkable for T1D patients to live more than one or two years after disease onset: one of the twentieth century’s utmost medical breakthroughs, insulin replacement, is still the mainstay of treatment for the vast majority of T1D patients today. That said, innovative ways of achieving improved insulin-mediated glycemic control are becoming accessible to patients, while tissue transplants, genetic modification and stem-cell therapies are showing promise in pre-clinical models and human trials in specific sub-groups of patients. In this section we will discuss the “old and new” of T1D therapies and moves towards personalization to increase treatment efficacy.
Insulin and combination drug therapies
By far, the most common T1D treatment approach is manual testing of blood sugar levels followed by sub-cutaneous injections of insulin, repeated throughout the day. Insulin pumps may be used in place of traditional injections [ 85 ]; these have the advantage of being able to continuously infuse small amounts of insulin sub-cutaneously, helping those patients with difficult-to-control glucose levels to better treat their disease. This is especially the case when coupled with continuous glucose monitoring (CGM) technology, which has been shown to improve control of blood glucose, thereby reducing long-term risks of diabetic complications [ 86 , 87 ].
Taking the combination of CGM and continuous insulin infusion to the next level is the advent of the artificial pancreas. By utilizing a CGM coupled via a control algorithm to an implanted insulin pump, people with T1D can achieve improved glycemic outcomes while reducing the burden of self-management [ 88 , 89 , 90 ]. A closed-loop artificial pancreas approach removes the need for the patients to manage their dosages at all, and some models also incorporate the pancreatic hormone glucagon, enabling glucose-responsive hormone delivery guided by real-time glucose sensor readings. This approach has the potential to accommodate highly variable day-to-day insulin/glucagon requirements. There will be a shift toward systems that offer more personalization, and individualization of adjusting parameters, glucose set algorithm aggressiveness proposed to be individualized including the daily targets [ 91 ] that can ensure tight glycemic control in affected patients [ 92 , 93 ]. Despite these advantages, still relatively few T1D patients are using an artificial pancreas, with the main obstacles being cost of the equipment, the need for a training infrastructure for users and clinicians, and a lack of clarity around which patient groups would benefit most from this technology (reviewed in [ 92 ]). In this case the technology has preceded the clinical sub-group analysis required to identify the patient groups who are most suited to the approach, calling for urgent research in order to fully exploit this important advance in insulin-replacement therapy.
Alongside developments in insulin replacement therapy, there has been a focus on identifying other drugs that can be combined with insulin to reduce hyper/hypoglycemia and improve metabolic variables without increasing adverse events (reviewed in [ 94 ]). Obese/T1D patients who predisposed to hypoglycemia and others with residual β-cell function could benefit from non-insulin antidiabetic drugs for future clinical trials [ 94 , 95 ]. Of these, promising candidates include metformin [ 96 ] and pramlintide, which have a role in glycemic control in both T1D and T2D and can modestly reduce triglyceride levels in T1D patients, as well as lowering hemoglobin A1c (HbA1 c ) and supporting weight loss [ 97 ]. In addition, glucagon-like peptide-1 receptor agonists (GLP-RAs) combined with insulin can reduce the daily bolus insulin dose required and improve glucose control and weight loss [ 98 ]. The incretins glucagon-like peptide 1 (GLP-1) is gut-derived hormone secreted upon food ingestion. The key physiological actions of GLP-1 are to accelerate nutrient-induced insulin release and inhibit glucagon secretion, in that way contributing to regulate postprandial glucose excursions [ 99 ]. In addition, other functions represented by inhibition of gastrointestinal motility and therefore works as “enterogastrone”, a hormone released by the lower gastrointestinal tract in reaction to lipids intake that constrains the caudal motion of the guts of chyme [ 100 ]. GLP-RAs used peripherally or centrally reduce food intake and escalate glucose-stimulated insulin secretion. The enzyme dipeptidyl peptidase-4 inhibitors (DPP-4) prevents the inactivation of GLP-1 and an adjunct therapy in a closed loop-system that can reduce postprandial blood glucose levels [ 101 ] and can significantly reduce the daily insulin dose but not the HbA1c level or the risk of hypoglycemia [ 102 ]. The DPP-4 enzyme is widely released in multiple organs and acts by cleavage of the two NH 2 -terminal amino acids of bioactive peptides if the second amino acid is alanine or proline [ 103 ]. It functions through affixed transmembrane fragment and a soluble protein. Both transmembrane fragment and soluble DPP-4 apply catalytic cleavage which alternatively inactivates peptides or generates new bioactive moieties that may exert competing or unique functions. Finally, sodium-glucose co-transporter inhibitors (SGLTi) are associated with improved glycemic control and a reduced insulin dosage leading to lower rate of hypoglycemic episodes [ 104 ]. In non-diabetics, approximately, 180 g of glucose is filtered diurnal through the renal glomeruli and is then re reabsorbed in the proximal convoluted tubule (PCT). This mechanism attained by inactive transporters, specifically, facilitated glucose transporters (GLUTs), and by active co-transporters, precisely, sodium-glucose co-transporters (SGLTs). SGLT1 and SGLT2 are considered most important out of the six identified SGLTs [ 105 ]. SGLTi acts by inhibiting SGLT2 in the PCT to block glucose reabsorption and ease its secretion in urine. The plasma glucose levels drop resulting in an improvement in the entire glycemic parameters [ 106 ].
In summary, traditional and combined approaches to insulin therapy remain important tools in the treatment of T1D, but they do not represent a cure and may not be able to achieve the level of glucose control necessary to avoid long-term complications arising from diabetes. Automated full closed-loop systems that can be programed to automatically manage meals may substantially benefit from faster acting insulins with a shorter duration of action. Proposing automatic flexibility to the individual’s changes not only daily patterns of insulin sensitivity but also to mechanically adjust to changes developing from illness, workout practices, eating routines and menstrual cycles. With the applications of machine learning (artificial intelligence), (AI), the future devices with the AI technologies could achieve the above relationship and to provide treatment suggestions and decisions based on the available data input. A unique and individualized predictive and decision support models using complex machine learning software and algorithms developed for insulin pumps for easier use and much more spontaneous daily life. Recently, Tyler et al. (reviewed in [ 107 ]) reported an algorithm for early recognition of unsafe insulin regimens which could be useful for improvement the glycemic results and minimize the dangerous complications of T1D [ 107 ]. Briefly, the algorithm offers weekly insulin dosage recommendations for adult patients with T1D using multiple daily injections protocol of long-acting basal and short-acting bolus insulin [ 108 ]. The hyperglycemia or hypoglycemia causes identification performed through validated single and dual hormone mathematical models that demonstrate a virtual platform of T1D patients [ 109 ]. The novel “virtual platform” employed to generate glucose observations used to train “decision making system”, which appeared to be in agreement with the endocrinologists’ decision of 67.9% when confirmed on actual human data [ 107 , 110 ]. In conclusion, such data provides guidance to physicians and T1D patients in effective use of insulin pumps data including but not limited to insulin dosing adjustments and other treatment decisions. It’s worth to mention how crucial that both physicians and diabetic patients understand the usefulness and limitations of insulin pumps and related treatment technologies. Sustaining the relationship between both will remain a critical factor in safe, thriving T1D treatment technology use.
- Gene therapy
Given the strong genetic component of T1D development, gene therapy offers a promising alternative to insulin injection for T1D treatment. Gene therapy is the procedure of transporting or manipulating genetic substances inside the cell as a therapeutic technique to cure disease [ 111 ]; it aims to modify faulty genes that are accountable for disease progression and thereby prevent disease onset or reverse its development (Fig. 3 ). The three key methodologies in gene therapy are: (I) introducing a new gene into the body (II) substituting defective genes with functional genes, and (III) deactivating the faulty genes triggering the disease [ 112 ]. Pre-clinical trials of gene therapy have now been tested with the aims of preventing or delaying onset of T1D, correcting insulin deficiency, promoting β-cell proliferation and survival, modulating the immune/inflammatory response or inducing insulin secretion by non-β cells (reviewed in [ 113 ]).
How genes are delivered to the human body during gene therapy approaches. Gene therapy have utilized two major approaches for transferring therapeutic transgenes into recipients 'body. First approach, is by direct infusion of the therapeutic gene into human body through a vehicle. Altered viruses often used for delivering the gene into specific human cell types. This method is inexact as it is limited to specific cell types that the viral vehicle can infect. Nonviral vehicles for directly delivering genes into cells are also being explored, including the use of plain DNA and DNA wrapped in a coat of fatty molecules known as liposomes. Th second approach utilize a living cells to transfer the therapeutic transgenes into recipients 'body. The transferring cells often a type of stem cell that removed from the body, and the therapeutic transgene is presented to them through direct transfer method. The genetically altered cells then grow and multiply before infused back to the recipient
Over the last few decades, gene transfer trials for the treatment of inherited or acquired diseases have mainly been performed in mice models. Non-obese diabetic (NOD) mouse has been the main animal model for studying autoimmune T1D. A key element of NOD model is the presence of spontaneous autoimmunity and T1D. The incidence of T1D is higher in females in NOD mice, [ 114 , 115 ], and is stated to have a minor prevalence in males in humans [ 116 , 117 ]. Like human, NOD mice develop autoantibodies and show elevated levels of autoreactive T-Cells ahead of disease onset [ 118 , 119 , 120 ]. The targeted antigens of β cell are also similar of both species, however, in the NOD mouse, the insulin seems to be the initiating antigen, while in human T1D, several antigens thought to be involved in this stage [ 118 ]. Gradual β cell death or malfunction, and autoimmune phenotypes shadowed by the onset of hyperglycemia exist in both human and NOD mouse [ 121 ], however, the appearance of pathogenic T cells have been noticed at 5-week-old NOD mice followed by insulitis throughout the pancreas by 12 weeks, reflecting the very aggressive nature of disease onset hits in shortened timeline (weeks only), compared to slower onset in humans (years after the autoantibodies appearance) [ 122 , 123 ].
The paradoxical assumption is that preventing T1D in NOD mice does not certainly convey what triggered the disease nor how to converse it. The NOD mouse model could be suitable to understand the genetic and immunologic features and causes of T1D including reversing the hyperglycemia when occurs. The model could serve as an approach to identify causative gene variants that can be tailored to discover novel therapeutic approaches for reversing new-onset T1D.
One particularly interesting strategy is the induced over-expression of insulin-like growth factor 1 (IGF1), which regulates immune functions and enhances the survival and proliferation of β-cells. Non-obese diabetic (NOD) mice spontaneously develop diabetes from around 10 weeks-of-age; however, when 4-week-old NOD mice underwent intra-ductal injection of an adeno-associated virus (AAV) encoding IGF1 to specifically transduce pancreatic cells, normoglycemia remained in 80% of these mice at week 28 [ 124 ]. Importantly, the same study also showed that treating NOD mice with the IGF1-encoding virus at 11 weeks-of-age, by which time significant β-cell destruction was evident, was able to re-establish lasting normoglycemia in 75% of mice [ 124 ].
In other animal studies, induced expression of regenerating islet-derived protein 3 gamma (Reg3g) has been reported to be able to regenerate β cells and preserve the cells despite autoimmune attacks [ 125 , 126 ]. Alongside, another study demonstrated the dynamic regulation of blood glucose levels in a model of T1D by stimulating the expression of glucose 6-phosphatase (G6Pase) in the liver of diabetic rats [ 127 ]. Here, expression of the G6Pase gene was induced by rising glucose levels and inhibited by insulin expression; in addition to achieving normoglycemia within a few hours of eating, no hypoglycemia was observed in the tested animals [ 127 ].
Gene therapy can also be used to induce insulin production in non-β-cells. Initial studies conducted on genetically engineered intestinal K cells [ 128 ] and hepatocytes showed that these cells were sensitive to glucose and could be induced to produce insulin. More recently, Jaen et al. demonstrated that a single injection of an AAV encoding insulin and glucokinase genes into skeletal muscle of diabetic dogs was able to induce metabolic normalization and normoglycemia lasting 8 years [ 129 ]. This study represents an important safety and efficacy step forwards for diabetes gene therapy, as although AAV vectors have been trialed in humans, their therapeutic use for gene transduction has yet to be tested clinically. There are concerns that transduced cells might be susceptible to recurring autoimmune attack, so enduring autoimmune protection must be demonstrated [ 130 , 131 ]. It is also possible that the viral vectors themselves might trigger an immune response that could worsen the disease condition [ 132 ], though Jaen et al. did not report any evidence of this in their study [ 129 ]. Modifications to the AAV vectors might hold some of the answers: in response to concerns that constitutive over-expression of insulin might risk hypo-glycaemia, one group has developed a Tet-off regulatable AAV vector for insulin expression that was able to both induce the expression of human insulin in diabetic mice, and be reversibly switched off to reduce insulin levels [ 133 ]. Thus, fine tuning of viral vectors combined with more long-term studies will be required to move towards vector-mediated reinstatement of insulin production in human patients.
In addition to induced insulin expression, several studies have looked at other targets implicated in T1D pathogenesis. For example, Klotho is an anti-aging gene that is expressed in pancreatic islets in mice [ 134 ] and humans [ 135 ]; a Klotho deficiency is linked with β-cell apoptosis, and reinstating its expression in mice under the control of a β-cell-specific promoter led to protection of β-cell function [ 134 ]. In human islet cells, treatment with the T1D drug gamma-aminobutyric acid in vitro significantly increased Klotho expression [ 136 ], indicating the possible clinical potential for this approach. A study by Flotyńska et al. demonstrated the relationship between fibroblast growth factor 23 (FGF23)/ Klotho system as a player in the human body metabolism, in addition to promoting longevity [ 137 ]. Despite the improvements in diabetes treatment, the long-term complications remain a big problem. The interesting correlation between the FGF23/Klotho system concentration and T1D management, duration, insulin resistance, and complications development require further attention and could be a predictor of cardiovascular risk in diabetic patients [ 138 ]. Combining gene therapy with immune modulation may also be promising. When NOD mice were pre-treated with anti-T-cell receptor β chain monoclonal antibody followed by hepatic gene therapy with Neurogenin-3 (which determines islet lineage) and the islet growth factor betacellulin, the researchers observed sustained induction of insulin-producing cells in the liver that achieved enduring reversal of new-onset or overt diabetes [ 139 ].
The discovery of β-cell mitogenic effects of ANGPTL8 (Angiopoietin Like 8), which was renamed “Betatrophin” to underline its effect on β cell replication, initially, created large interest but consequently, have been subjected to substantial debate regarding its anticipated mitogenic effects [ 140 ]. The initial findings proposed that the over expression of ANGPTL8 in mice model stimulated a 17-fold increase in pancreatic β-cell proliferation [ 140 , 141 ]. Consequent research studies in mice disputed this statement as no substantial evidence could be observed to support the direct effects of ANGPTL8 on beta-cell proliferation [ 140 , 142 , 143 ], Therefore, ANGPTL8 is not considered as a potential agent for diabetes intervention although some reports supported the initial observations in rats [ 144 ]. In a study performed by Chen et al. (reviewed by [ 144 ]), targeted gene delivery approach has been used to deliver human ANGPTL8 gene plasmids to different organs of normal adult rats including the pancreas, liver and skeletal muscles and compared the efficiency of beta β cell replication induced by ANGPTL8 gene using the rat model of streptozotocin (STZ)-induced diabetes. The improvement in glucose tolerance plus the elevated fasting plasma insulin levels were directly associated with β cell proliferation. A novel gene therapy technique used here through targeting the transfer of non-viral DNA to the pancreatic islet by using ultrasound-targeted microbubble destruction (UTMD) beside an altered insulin promoter [ 140 , 145 ]. UTMD considered as promising method for target-specific gene delivery, and it has been successfully investigated for the treatment of many diseases in the past decade including cardiovascular disorders and cancer.
A novel approach to gene therapy for T1D involves targeting post-transcriptional modifications that give rise to pathogenic splice variants. Cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4) is an immune-modulatory protein where expression of different forms has been linked to T1D susceptibility or resistance in T1D patients [ 146 ] and some other autoimmune diseases [ 147 ]. To modulate the immune response leading to T1D onset, Mourich et al. employed an antisense-targeted splice-switching approach to produce CTLA-4 splice forms in NOD mouse T-cells [ 148 ]. In this study, when the antisense approach was used to mask pre-mRNA splice recognition sites and redirect the splicing machinery to skip selected exons, induced over-expression of the protective ligand-independent form of CTLA-4 protected NOD mice from disease [ 148 ].
Lastly, while these studies clearly indicate the exciting potential of in vivo gene therapy, the process remains complex, in addition, the possible toxicity of the viral vectors and the improvements needed to the delivery systems to achieve the maximum levels of gene expression still under development [ 125 ]. That said, twenty gene and cell-based gene therapy products have now been licensed for the treatment of human cancers and monogenic disorders “e.g., Neovasculgen (Vascular endothelial growth factor, VEGF), Glybera (lipoprotein lipase, LPL S447X gene), Defitelio (single-stranded oligonucleotides-VOD), Rexin-G (Retroviral vector encoding cyclin G1 inhibitor), Onpattro (RNAi-transthyretin gene)” and clinical trials in these diseases are ongoing [ 149 ]. There is real hope that effective approaches to direct gene therapy for T1D patients, particularly those with monogenic T1D, will be developed in the near future, building on its success in other conditions.
Stem cell therapies
Perhaps the most promising innovation in T1D therapy has been the exploration of the potential of stem cells. This unique population is able to self-renew indefinitely, form single cell-derived clonal cell populations, and differentiate into various cell types [ 150 ]. Stem cells from diverse sources have now been investigated for their potential in β-cell regeneration, as discussed below.
Embryonic stem cells
Embryonic Stem Cells (ESCs) are derived from the undifferentiated inner cell mass of human embryos and have the advantage of being completely pluripotent. Several different approaches to generating insulin-producing cells (IPCs) from ESCs have been explored. Human Embryonic Stem Cells ESCs (hESCs) in feeder-free cultures avoid the risk of animal pathogen transfer and are readily scalable, making this approach best-suited to clinical use [ 151 ].
Kroon et al. instructed the differentiation of hESCs by directly overexpressing essential β-cell transcription factors (TFs) including Pancreatic and Duodenal Homeobox 1 (PDX1), SRY-Box Transcription Factor 9 (SOX9), Homeobox protein Nkx-6.1 (NKX6.1) and Neurogenin 3 (NGN3; following engraftment into diabetic mice, the resulting cells recapitulated key features of pancreatic β-cells and protected against hyperglycemia [ 152 ]. Subsequently, an important step forwards in the use of hESCs for T1D therapy occurred when scientists from the University of British Columbia developed a seven-stage protocol that efficiently converted hESCs into IPCs. This protocol generated endocrine cells with insulin content similar to that of human islet cells and that were capable of glucose-stimulated insulin secretion in vitro as well as rapid reversal of diabetes in vivo in mice [ 153 ]. Additional studies have highlighted the possible roles of other growth and extracellular matrix factors, including laminin, nicotinamide, insulin [ 154 ], and retinoic acid [ 155 ] in the generation of IPCs from ESCs, but these findings have yet to be integrated into a combined approach suitable for clinical use.
hESCs also have the potential to generate cells uniquely tailored for the recipient. Recently, Sui et al. showed that transferring the nucleus of skin fibroblasts from T1D patients into hESCs gave rise to differentiated β-cells with comparable performance to naturally occurring β-cells when transplanted into mice [ 156 ].
Despite the promise of hESCs, great concern around their potential to initiate teratomas has largely limited their clinical exploration in T1D. However, Qadir et al. recently demonstrated a means of overcoming this risk: the authors modified hESCs to include two suicide gene cassettes, whose expression results in cell death in the presence of specific pro-drugs [ 157 ]. Their method is designed to provide a double fail-safe control, such that I) only IPCs survive selection; and II) cells that may de-differentiate after transplantation can be eliminated. Furthermore, ensuring that undifferentiated cells are sensitive to two pro-drugs makes it less likely than any tumorigenic cells would survive or became resistant [ 158 ].
Human pluripotent stem cells
Naturally, Human Pluripotent Stem Cells (hPSCs) are immature cells that have the capacity to become nearly any cell type in the body. Accordingly, there has been much research interest in using them to regenerate a wide range of tissues, including the pancreas. Under the control of specific growth factors, signaling pathways and activating/inhibitory molecules [ 159 , 160 ] the steps of pancreatic cell differentiation have been successfully recreated in vitro.
The importance of this approach is its potential to generate a ready supply of in vitro-differentiated β-cells for transplantation into T1D patients. Recent studies have reported the successful differentiation of β-like cells with enhanced function from pancreatic progenitors through modulating Epidermal growth factor beta (EGF-β) signaling and cellular cluster size, giving rise to stem cell-derived β-cells with the ability to express key β-cell markers and insulin [ 161 , 162 ]. What remains unclear is how well these in vitro-derived cells will function in vivo , but this is nonetheless a promising first step.
Hematopoietic stem cells
Taking a different approach, myeloablation coupled with autologous Hematopoietic Stem Cells (HSCs) transplantation aims to halt the autoimmune destruction of the pancreas and reestablish tolerance. The first autologous HSCs transplantation in a T1D patient was executed by the Voltarelli’ group in 2007: 15 patients aged between 14 add 31 years, and with recent T1D onset (previous 6 weeks) diagnosed by clinical findings, hyperglycemia and GAD65 autoantibodies were involved in the study [ 163 ]. When these patients were treated with autologous HSCs, most achieved insulin independence with good glycemic control lasting until the final 29.8-month follow-up, together with a notable increase in β-cell function [ 164 ]. Autologous HSC transplantation has also been used successfully to treat diabetic sequelae, including vascular complications [ 165 ] and retinopathy [ 166 ]. Other studies have focused on understanding the mechanisms underlying successful HSCs transplantation in T1D: for example, Ye et al., found that autologous HSC treatment was associated with the inhibition of T-cell proliferation and pro-inflammatory cytokine production [ 167 ]; while Xiang et al. uncovered a critical role for the remaining functional β-cells on the autologous transplant of HSCs [ 168 ].
Despite the evident successes of autologous HSCs transplantation for T1D, various complications can occur, ranging from relatively mild symptoms such as febrile neutropenia, nausea, and alopecia to more severe complications such as de novo autoimmunity and systemic infections, which in one case resulted in death [ 169 , 170 ]. The development of new strategies involving autologous HSCs therapy for newly-diagnosed T1D patients coupled with appropriate and effective use of immunosuppressive drugs will be crucial to maximize the frequency and function of T and B regulatory cells, while minimizing the activity of autoreactive islet-specific T and B memory cells. In this way, we should be able to improve treatment outcomes in T1D patients undergoing transplantation.

Mesenchymal stem cells
Mesenchymal Stem Cells (MSCs) are multi-potent stromal cells able to differentiate in vitro into a range of cell types; characteristically adipocytes, chondrocytes, myocytes, and osteoblasts [ 171 ]. MSCs are relatively easy to isolate from different sources in the body and numerous studies have assessed their use in T1D therapy.
Historically, the bone marrow has been the main source of MSCs [ 172 ]. Xie et al. first trialed generating IPCs from T1D patients’ bone marrow MSCs (BM-MSCs) and showed the co-expression of insulin and C-peptide in cells injected into diabetic mice, leading to attenuated hyperglycemia [ 173 ]. Alongside, genetically-modified human BM-MSCs expressing VEGF and PDX1 reversed hyperglycemia in more than half of diabetic mice and enabled survival and weight maintenance in all animals [ 174 ]. These promising pre-clinical results led to human trials: when BM-MSCs were injected into the splenic artery of T1D patients, they induced an increase in C-peptide levels that was maintained for 3 years; unfortunately, this had no significant effects on glycemic control due to insufficient production of insulin by the grafted cells [ 175 ]. Since then, new methods have been developed aiming to improve in vivo outcomes. For example, Zhang et al. co-cultured BM-MSCs with pancreatic stem cells which led the MSCs to adopt a pancreatic islet morphology; when these cells were injected into diabetic rats they attenuated glycated albumin levels and significantly increased serum insulin and C-peptide [ 176 ].
The main disadvantage of BM-MSCs is the difficulty in isolating the cells and the morbidity associated with the procedure. These issues led to interest in the use of Muscle-Derived Stem/Progenitor Cells (MDSPCs), which exist in skeletal muscle and have the capacity for long-term proliferation, are resistant to oxidative and inflammatory stress, and show multi-lineage differentiation potential [ 177 ]. To investigate the therapeutic potential of autologous MDSPCs transplantation for T1D, Lan et al. applied a four-stage MDSPCs differentiation protocol to generate IPCs in vitro and injected them into diabetic mice: these β-cell-like-cells effectively improved hyperglycemia and glucose intolerance and increased the survival rate in diabetic mice without the use of immunosuppressants [ 178 ].
Building on the promise of BM-MSCs and MDSPCs, researchers sought an equally potent but more abundant and easily accessed source of stem cells. Adipose-Derived Stem Cells (ADSCs) have recently been explored for T1D treatment, and have the advantage over MDSPCs of being readily accessible and harvested, even in older patients [ 179 ]. IPCs differentiated from ADSCs show significant expression of β-cell markers, insulin and c-peptide following transfer into diabetic mice [ 180 ]. In 2019, IPCs derived from ADSCs using a novel three-dimensional (3D) xenoantigen-free protocol were shown to exhibit key features of pancreatic β cells in vitro and differentiated into IPCs in diabetic nude mice in vivo [ 181 ]. Another study showed the potential for combining ADSCs treatment with gene therapy by transducing ADSCs with a furin-cleavable insulin gene (INS-FUR), which led to enhanced insulin expression in the differentiated adipocytes, and alleviated hyperglycemia in diabetic mice [ 182 ].
Removing the need for adult stem cell donors completely, the umbilical cord is now used as a successful alternative stem cell source for regenerative medicine. Umbilical cord blood (UCB) is rich in HSCs, can be easily harvested without the need for interventions, and also contains a large number of naive functioning T-regulatory cells (Treg) with the potential to reduce autoimmunity [ 183 , 184 ]. Moreover, the MSCs within UCB (UCB-MSCs) have high proliferative capacity, are easily bankable and have low tumorigenicity [ 185 ]. Together these features are making UCB-MSCs the preferred option for potential T1D cell-based therapies. Studies in animal models have showed encouraging results: when Prabakar et al. adapted an ESC protocol for IPC culture and applied it to UCB-MSCs they generated expanded populations of undifferentiated IPCs expressing the key pancreatic TFs PDX1, NGN3, Neuronal Differentiation 1 (NEUROD1), NKX6.1, and Insulin Gene Enhancer Protein ISL-1 “ISL LIM Homeobox 1” (ISL1) [ 186 ]. Following transplantation into mice, these cells subsequently differentiated into glucose-responsive IPCs [ 186 ]. Zhao et al. took a different approach to exploiting stem cells for T1D treatment, instead focusing on their capacity to downregulate immune responses. The authors achieved reversal of the autoimmune response in NOD mice by transferring autologous Tregs that had been co-cultured with human UCB-MSCs; this led to increased insulin secretion, reduced hyperglycemia and preservation of islet architecture [ 187 , 188 , 189 ].
Despite promising signs in rodent studies, the potential of UCB-MSCs treatment for T1D in humans has yet to be fully realized. Haller et al. attempted the first autologous UCB-MSCs transplantation in recently-diagnosed T1D patients in 2008: early indications were encouraging, with transplanted patients showing slowed loss of endogenous insulin production and an increase in peripheral blood Treg cells after 6 months [ 190 ]. However, a subsequent study by the same group found no significant difference in C-peptide levels after autologous transfusion of UCB-MSCs combined with oral docosahexaenoic acid and vitamin D supplementation [ 191 ]. Similarly, in a non-randomized controlled trial in seven new-onset T1D children who underwent autologous UCB-MSCs infusion, there was no evidence of improvements in metabolic regulation or immune function at the one-year follow-up [ 192 ].
The possible reasons for the failure of UCB-MSCs to effectively halt the autoimmune progression in human subjects’ trials, could be the inadequate number of cells with immunomodulation capacity being transferred to T1D patients, or due to the ongoing autoimmune reactions especially in new-onset T1D patients that may comprise memory T-cells, refractive to regulation by Tregs, that enhance the autoimmune destruction of β-cells [ 193 ]. Merging transient immune depletion agents with consequent infusion of expanded UCB Tregs may effectively balance the environment of Tregs and effector T cells in T1D patients. Finally, more controlled and randomized clinical trials are crucial to further improve the transplantation process and to investigate the mechanism of UB-MSC survival and behavior in live bodies overtime. Further investigations with larger sample sizes will be important to understand how to translate the successful application of UCB-MSCs infusion from mouse to human.
Cord blood is not the only source of stem cells within the human umbilical cord; Wharton’s jelly is a mucoid connective tissue in the umbilical cord that can also serve as a source of clinically-relevant MSCs (Wharton’s jelly-derived mesenchymal stem cells, WJ-MSCs) for both IPC derivation and immunosuppression [ 194 ]. Briefly, WJ-MSCs collection occurs at the time of delivery and avoids the known adverse effects associated with adult stem cell collection from the bone marrow or adipose tissue. Furthermore, features including a high WJ-MSCs proliferation rate, an immune privileged status, minimal associated ethical concerns, and non-tumorigenic capacity render these cells an excellent option to be used in regenerative medicine applications [ 195 ].
One of the first studies to use β-cell-like cells derived from WJ-MSCs tested their effects following transplantation into patients with new-onset T1D [ 196 ]. Interestingly, a concurrent study suggested that the WJ-MSCs might restore the function of β-cell in T1D patients but it could be affected by the patient’s ketoacidosis history [ 197 ], though the underlying mechanism to support this has not yet been tested. A genetically and chemically combined approach for WJ-MSCs induction into IPCs has also been shown to improve the cells’ homing efficiency to the pancreatic gland of diabetic rats [ 198 ]; taken together with a growing body of clinical data, these findings may help optimize the use of differentiated WJ-MSCs in T1D.
Undifferentiated WJ-MSCs also have the capacity to induce a protective immune-suppressive state in animal models of T1D and in patients. A study in mice performed by Tsai et al. showed that undifferentiated WJ-MSCs implanted into NOD mice both differentiated into IPCs in vivo, leading to islet repair and maintaining levels of C-peptide and insulin production, and induced beneficial immunosuppression [ 199 ]. Such evidence in rodents has since led to the initiation of human trials. A safety and dose-escalation trial is ongoing: in the first stage, Carlsson et al. are carrying out WJ-MSCs allotransplantation into newly-diagnosed (< 2 years) T1D adult men with dose-escalation to establish safety parameters; in the second double-blinded, parallel, placebo-controlled stage, a cohort of T1D patients (men and women) will undergo WJ-MSCs allotransplantation aiming to achieve immunosuppression and preserve endogenous insulin production [ 200 ]. Altogether, comparing WJ-MSCs, UCB-MSCs [ 201 ] and BM-MSCs [ 202 ], it seems that WJ-MSCs are the better anti-diabetic agents, being more homogenous and having greater potential to initiate pancreatic regeneration.
Medical nutrition therapy in managing T1D
A healthy lifestyle including eating pattern beside pharmacotherapy are major components of managing T1D. For many diabetic patients, determining what to eat is the most challenging part of the treatment plan. Effectual nutrition therapy interventions may be an element of a comprehensive T1D education package or an individualized session [ 203 ]. Furthermore, T1D individuals on multiple daily insulin doses, the main focus for nutrition therapy must be on how to adjust insulin doses based on scheduled carbohydrate intake [ 204 , 205 ]. Reported HbA1 C from medical nutrition therapy (MNT) decreases are similar or greater than what would be expected with currently available pharmacologic therapies for T1D [ 206 ]. Rigorous insulin management education programs that include MNT have been shown to reduce HbA1 C up to 1.9% at 3–6 months, in addition to significant improvement in quality of life over the time [ 203 , 207 ]. There is no “one-size-fits-all” eating pattern that could work collectively for all T1D individuals, nutritional therapy should be individualized and supervised under the care of a dietitian based on the heath goals, personal favorites and access to healthy options should be considered [ 208 , 209 ].
Remaining obstacles and future directions
Marked progress has been made in the past decade towards both personalized diagnosis and treatment for T1D, but significant obstacles and research gaps remain between the current state of knowledge and its translation into widespread clinical benefit. As in many other diseases, the precision medicine for T1D is a new and growing field. Increases ethical, social and legal issues and the necessity to find precise ways to protect subjects’ privacy and confidentiality of their health data. In addition, patients need to know and understand the associated risks and expected benefits of being part of precision medicine research, which requires researchers to create a meticulous approach of obtaining informed consent to recruit participants to research studies. Furthermore, cost-effectiveness of precision medicine approaches comparing to the current standard of care is a gap that needs to be resolved. The impact of diabetes on healthcare systems has been evaluated as the largest contributor to entire healthcare costs. For example, in a study performed by Stedman et al. (reviewed in [ 34 ]), the differences between T1D/T2D and non-diabetes subjects in connection to hospital and associated costs in in England. In summary, T1D individuals demanded five times additional secondary care support than non-diabetes subjects. The analysis shows that extra cost of running of hospital services due to their diabetes comorbidities is £3 billion over that for non-diabetes, within this figure, T1D has three times as much cost impact as T2D, suggesting that supporting patients in diabetes management may considerably decrease hospital activity, in addition, the possibility and potential for precision treatment in diabetes is massive, yet profound understanding is missing. It will be vital to decide when and how the application of therapeutics in precision diabetes medicine improves outcomes in a cost-effective style.
Much of our current knowledge of personalized therapeutic approaches to treat T1D comes from experiments in animal models; but a recurring theme in the T1D therapy field is the lack of translation between promising results in mice and the same outcome in humans. Mice are most commonly used for these experiments but exhibit both macroscopic and microscopic differences in pancreatic physiology and T1D pathophysiology. For example, rodents islets have a distinct core structure comprising 60–80% β-cells, 15–20% α- cells, < 10% δ-cells and < 1% PP cells [ 210 , 211 , 212 ]; while human islets tend to have ~ 50% β-cells, ~ 40% α- cells, ~ 10% δ-cells and < 5% P-cells [ 213 , 214 ]. In addition, notable differences in the repertoire of receptors and long non-coding RNAs between mouse and human beta cells have been identified [ 215 ]. In terms of modeling T1D, the NOD mouse has long been the approach of choice for majority of pre-clinical and translational invasive studies [ 216 ]. The main strength of the NOD mouse is the presence of spontaneous autoimmunity leading to T1D [ 118 , 216 ] however, in the mice, this is triggered by the insulin antigen, while in humans this phenomenon is more complex, involving several inducing antigens followed by hyperglycemia [ 217 , 218 ]. Taken together, extreme caution must be exercised when attempting to draw conclusions from animal models and apply them to the human situation [ 219 ].
Despite advances in the various therapies discussed above, an ongoing challenge in T1D treatment is the extreme heterogeneity in patients’ disease triggers, prognosis, pathological pathways and thus the response to treatment [ 220 , 221 , 222 , 223 ]. Important research in human populations has revealed previously unappreciated heterogeneity within the T1D patient population. This has two major implications: firstly, that we are unlikely to discover a “one-size-fits-all” therapy able to cure every case; and secondly that personalized diagnosis is a necessary pre-requisite for personalized treatment. The first step towards this will be the routine assessment of T1D subtype in newly diagnosed patients, including screening for monogenic T1D as well as autoantibody testing to distinguish idiopathic T1D, and, in future, genetic profiling to inform potential gene therapy or stem cell approaches.
In diabetes, the precision medicine approach has been inspired by work including that of Zhao et al., who first developed stem cell educator therapy where T1D patients’ lymphocytes are briefly separated from the blood and co-cultured with UC-MSCs within a closed-loop-system, before being returned to the patient; this treatment dramatically improved metabolic control, reversed autoimmunity and promoted β-cell regeneration [ 143 ]. Al-Anazi et al. used a similar approach to try and treat multiple myeloma in 45 adults with T1D who had undergone autologous HSCs; surprisingly the patients were also cured of their diabetes and became insulin-independent [ 144 ].
In fact, the next step towards stem-cell-mediated precision medicine for T1D is likely to involve the incorporation of gene therapeutic approaches, synergizing existing stem cell knowledge with advances in cellular and genetic engineering techniques, such as nuclear transfer and genome editing. Moreover, an emerging understanding of the TFs and epigenetic processes that control pancreatic islet lineage-commitment [ 224 ], as well as the role of microRNAs in driving cell lineage differentiation [ 225 ] are beginning to unlock new knowledge on T1D pathogenesis [ 226 , 227 ], and are opening fresh possibilities in β-cell generation [ 228 , 229 , 230 ].
Together these factors can all be used towards designing a successful protocol for precision medicine in T1D. Alongside, the reframing of T1D as primarily a metabolic disorder (rather than an autoimmune condition) that reflects the combined genomic and environmental landscape of the patient, has facilitated the discovery of new therapeutic targets and diagnostic/prognostic biomarkers [ 231 , 232 ]. Finally, the ongoing discovery of new and important influences on diabetic pathology, such as the role of gut microbiota [ 233 ], and the latest perceptions into the mechanism of T1D and the accumulated recent data that being translated into prospects for tissue-specific prevention trials toward eliminating progressive β-cell loss [ 234 ], continues to add to our understanding of this important disease, and thereby our ability to rationally design and test novel interventions with the promise of the future eradication of T1D.
Availability of data and materials
Not applicable.
Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am. 2005;52(6):1553–78.
Article PubMed Google Scholar
Patterson CC, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–17.
Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes–the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–403.
Article CAS PubMed Google Scholar
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23(10):1516.
Saeedi P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:48.
Article Google Scholar
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med. 2008;359(26):2849–50.
Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–9.
Barrett JC, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–7.
Article CAS PubMed PubMed Central Google Scholar
Sharp SA, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care. 2019;42(2):200.
Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90(1):7–24.
Steck AK, et al. Association of Non-HLA Genes With Type 1 Diabetes Autoimmunity. Diabetes. 2005;54(8):2482.
Nisticò L, et al. The CTLA-4 gene region of chromosome 2q33 is linked to, and associated with, type 1 diabetes. Hum Mol Genet. 1996;5(7):1075–80.
Bottini N, Vang T, Cucca F, Mustelin T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol. 2006;18(4):207–13.
Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57(2):176–85.
Clayton DG. Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet. 2009;5(7):e1000540.
Article PubMed PubMed Central CAS Google Scholar
Wray NR, Yang J, Goddard ME, Visscher PM. The genetic interpretation of area under the ROC curve in genomic profiling. PLoS Genet. 2010;6(2):e1000864.
Ilonen J, Hammais A, Laine AP, Lempainen J, Vaarala O, Veijola R, Simell O, Knip M. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62(10):3636–40.
Krischer JP, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–7.
Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290(13):1713–20.
Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia. 2015;58(6):1188–97.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet (London, England). 2016;387(10035):2340–8.
Article CAS Google Scholar
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318(7):647–56.
Grammatiki M, Rapti E, Karras S, Ajjan R, Kotsa K. Vitamin D and diabetes mellitus: Causal or casual association? Rev Endocr Metab Disord. 2017;18(2):227–41.
Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12(3):154.
Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and type 1 diabetes. N Engl J Med. 2004;350(14):1398–404.
Butalia S, Kaplan GG, Khokhar B, Rabi DM. Environmental risk factors and type 1 diabetes: past, present, and future. Canad J Diabetes. 2016;40(6):586–93.
Kolb H, Elliott R. Increasing incidence of IDDM a consequence of improved hygiene? Diabetologia. 1994;37(7):729–729.
Baschal EE, Eisenbarth GS. Extreme genetic risk for type 1A diabetes in the post-genome era. J Autoimmun. 2008;31(1):1–6.
Wang Z, Xie Z, Lu Q, Chang C, Zhou Z. Beyond genetics: what causes type 1 diabetes. Clin Rev Allergy Immunol. 2017;52(2):273–86.
Mehers KL, Gillespie KM. The genetic basis for type 1 diabetes. Br Med Bull. 2008;88(1):115–29.
Craig ME, Kim KW, Isaacs SR, Penno MA, Hamilton-Williams EE, Couper JJ, Rawlinson WD. Early-life factors contributing to type 1 diabetes. Diabetologia. 2019;62(10):1823–34.
Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15(11):635–50.
Chua K-P, Lee JM, Conti RM. Out-of-pocket spending for insulin, diabetes-related supplies, and other health care services among privately insured US patients with type 1 diabetes. JAMA Internal Med. 2020;180(7):1012–4.
Stedman M, et al. Cost of hospital treatment of type 1 diabetes (T1DM) and type 2 diabetes (T2DM) compared to the non-diabetes population: a detailed economic evaluation. BMJ Open. 2020;10(5):e033231.
Article PubMed PubMed Central Google Scholar
Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the US: a propensity score matching method. PloS one. 2010;5(7):e11501–e11501.
Insel RA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–74.
Ziegler AG, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9.
Insel R, Dutta S, Hedrick J. Type 1 diabetes: disease stratification. Biomedicine Hub. 2017;2:1–16.
Ludvigsson J, Heding L, Larsson Y, Leander E. C-peptide in juvenile diabetics beyond the postinitial remission period relation to clinical manifestations at onset of diabetes remission and diabetic control. Acta Pædiatrica. 1977;66(2):177–84.
Control, D. and C.T.R. Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–23.
Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832–6.
Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund J-YC, Lachin JM. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45–53.
Larsson HE, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–52.
Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–404.
Dabelea D, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133(4):e938–45.
Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308–13.
Lundgren M, et al. Reduced morbidity at diagnosis and improved glycemic control in children previously enrolled in DiPiS follow-up. Pediatr Diabetes. 2014;15(7):494–501.
Krischer JP. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia. 2013;56(9):1919–24.
Orban T, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9.
Ferrat LA, et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med. 2020;26(8):1247–55.
Sosenko JM, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31(3):528–33.
Rubio-Cabezas O, Hattersley AT, Njølstad PR, Mlynarski W, Ellard S, White N, Chi DV, Craig ME. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2014;15(S20):47–64.
Bonifacio E, et al. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med. 2018;15(4):e1002548.
Redondo MJ, et al. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care. 2018;41(9):1887–94.
Patel KA, Oram RA, Flanagan SE, De Franco E, Colclough K, Shepherd M, Ellard S, Weedon MN, Hattersley AT. Type 1 diabetes genetic risk score: a novel tool to discriminate monogenic and type 1 diabetes. Diabetes. 2016;65(7):2094–9.
Wilson RM, Messaoudi I. The impact of maternal obesity during pregnancy on offspring immunity. Mol Cell Endocrinol. 2015;418:134–42.
Arabin B, Baschat AA. Pregnancy: An Underutilized Window Of Opportunity To Improve Long-Term Maternal And Infant Health—An Appeal For Continuous Family Care And Interdisciplinary Communication. Front Pediat. 2017;5:69.
Google Scholar
Organization WH. WHO recommendations non-clinical interventions to reduce unnecessary caesarean sections. Berlin: World Health Organization; 2018.
Antvorskov JC, et al. Association between maternal gluten intake and type 1 diabetes in offspring: national prospective cohort study in Denmark. BMJ (Clinical research ed). 2018;362:k3547–k3547.
Fronczak CM, Barón AE, Chase HP, Ross C, Brady HL, Hoffman M, Eisenbarth GS, Rewers M, Norris JM. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237–42.
Penno, M.A.S., J.J. Couper, M.E. Craig, P.G. Colman, W.D. Rawlinson, A.M. Cotterill, T.W. Jones, L.C. Harrison, and E.S. Group. Environmental determinants of islet autoimmunity (ENDIA): a pregnancy to early life cohort study in children at-risk of type 1 diabetes. BMC Pediatr. 2013;13(1):124.
Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167(9):800–7.
Couper JJ, Beresford S, Hirte C, Baghurst PA, Pollard A, Tait BD, Harrison LC, Colman PG. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care. 2009;32(1):94–9.
Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, Rewers M, Norris JM. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediat. 2013;167(9):808–15.
Lamb MM, Miller M, Seifert JA, Frederiksen B, Kroehl M, Rewers M, Norris JM. The effect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Pediatr Diabetes. 2015;16(1):31–8.
Porcelli B, Pozza A, Bizzaro N, Fagiolini A, Costantini MC, Terzuoli L, Ferretti F. Association between stressful life events and autoimmune diseases: a systematic review and meta-analysis of retrospective case-control studies. Autoimmun Rev. 2016;15(4):325–34.
Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol. 2010;6(5):279.
Lamb MM, Yin X, Zerbe GO, Klingensmith GJ, Dabelea D, Fingerlin TE, Rewers M, Norris JM. Height growth velocity, islet autoimmunity and type 1 diabetes development: the Diabetes Autoimmunity Study in the Young. Diabetologia. 2009;52(10):2064–71.
Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89(6):2526–39.
Peiris H, Bonder CS, Coates PTH, Keating DJ, Jessup CF. The β-cell/EC axis: how do islet cells talk to each other? Diabetes. 2014;63(1):3.
Yurkovetskiy LA, Pickard JM, Chervonsky AV. Microbiota and autoimmunity: exploring new avenues. Cell Host Microbe. 2015;17(5):548–52.
Group, T.S. The environmental determinants of diabetes in the young (TEDDY) Study. Ann N Y Acad Sci. 2008;1150:1–13.
Chakhtoura M, Azar ST. The role of vitamin D deficiency in the incidence, progression, and complications of type 1 diabetes mellitus. Int J Endocrinol. 2013;2013:148673.
Pereira PF, Alfenas R, Araújo RMA. Does breastfeeding influence the risk of developing diabetes mellitus in children? A review of current evidence. J Pediat (English Edition). 2014;90(1):7–15.
Norris JM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420–8.
Uusitalo U, et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY Study. JAMA Pediatr. 2016;170(1):20–8.
Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Åkerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(suppl 2):S125.
Infante M, et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019;11(9):2185.
Article CAS PubMed Central Google Scholar
Stene LC, Gale EAM. The prenatal environment and type 1 diabetes. Diabetologia. 2013;56(9):1888–97.
Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, Hattersley AT, Weedon MN. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337–44.
Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6(2):122–9.
Thomas NJ, Lynam AL, Hill AV, Weedon MN, Shields BM, Oram RA, McDonald TJ, Hattersley AT, Jones AG. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia. 2019;62(7):1167–72.
Franks PW, Pomares-Millan H. Next-generation epidemiology: the role of high-resolution molecular phenotyping in diabetes research. Diabetologia. 2020;63(12):2521–32.
Elding Larsson H, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15(2):118–26.
McAdams BH, Rizvi AA. An overview of insulin pumps and glucose sensors for the generalist. J Clin Med. 2016;5(1):5.
Article PubMed Central CAS Google Scholar
Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371–8.
Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, Longo M, Giugliano D, Esposito K. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146.
Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579–89.
Sherr JL, Tauschmann M, Battelino T, de Bock M, Forlenza G, Roman R, Hood KK, Maahs DM. ISPAD clinical practice consensus guidelines 2018: diabetes technologies. Pediatr Diabetes. 2018;19:302–25.
DeSalvo DJ, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271–5.
Lal RA, Ekhlaspour L, Hood K, Buckingham B. Realizing a closed-loop (artificial pancreas) system for the treatment of type 1 diabetes. Endocr Rev. 2019;40(6):1521–46.
Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36(3):279–86.
Choi SB, Hong ES, Noh YH. Open artificial pancreas system reduced hypoglycemia and improved glycemic control in patients with type 1 diabetes. Diabetes. 2018;67:964.
Frandsen CS, Dejgaard TF, Madsbad S. Non-insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol. 2016;4(9):766–80.
Ahrén B, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39(10):1693–701.
Article PubMed CAS Google Scholar
Meng H, Zhang A, Liang Y, Hao J, Zhang X, Lu J. Effect of metformin on glycaemic control in patients with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2018;34(4):e2983.
Hoogwerf BJ, Doshi KB, Diab D. Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vasc Health Risk Manag. 2008;4(2):355–62.
Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017;8(4):727–38.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39.
Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283–212283.
Underland LJ, Ilkowitz JT, Katikaneni R, Dowd A, Heptulla RA. Use of sitagliptin with closed-loop technology to decrease postprandial blood glucose in type 1 diabetes. J Diabetes Sci Technol. 2017;11(3):602–10.
Guo H, Fang C, Huang Y, Pei Y, Chen L, Hu J. The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;121:184–91.
Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35(6):992–1019.
Dellepiane S, BenNasr M, Assi E, Usuelli V, Letizia T, Addio F, Zuccotti GV, Fiorina P. Sodium glucose cotransporters inhibitors in type 1 diabetes. Pharmacol Res. 2018;133:1–8.
Whalen K, Miller S, Onge ES. the role of sodium-glucose co-transporter 2 inhibitors in the treatment of type 2 diabetes. Clin Ther. 2015;37(6):1150–66.
Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Therapy. 2014;5(2):355–66.
Tyler NS, et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat Metabol. 2020;2(7):612–9.
Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8.
Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG. A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PLoS ONE. 2019;14(7):e0217301.
Cover T, Hart p. Nearest Neighbor Pattern Classification (1967) journal= The Edison Foundation Institute for Electric Efficiency. The Edison Foundation Institute for Electric Efficiency, p. 21–27.
Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: As good as it gets? Nat Med. 1999;5(6):601–4.
Kaufmann KB, Büning H, Galy A, Schambach A, Grez M. Gene therapy on the move. EMBO Mol Med. 2013;5(11):1642–61.
Chellappan DK, et al. Gene therapy and type 1 diabetes mellitus. Biomed Pharmacother. 2018;108:1188–200.
Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Exp Anim. 1980;29(1):1–13.
Makino S, Muraoka Y, Kishimoto Y, Hayashi Y. Genetic analysis for insulitis in NOD mice. Exp Anim. 1985;34(4):425–31.
Group, E.A.S. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355(9207):873–6.
Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harbor perspectives in medicine. 2012;2(11):7641.
Melanitou E, Devendra D, Liu E, Miao D, Eisenbarth GS. Early and quantal (by litter) expression of insulin autoantibodies in the nonobese diabetic mice predict early diabetes onset. J Immunol. 2004;173(11):6603–10.
You S, et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54(5):1415–22.
Gregori S, Giarratana N, Smiroldo S, Adorini L. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J Immunol. 2003;171(8):4040–7.
DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proc Natl Acad Sci. 1998;95(21):12538–43.
Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–31.
Leete P, Willcox A, Krogvold L, Dahl-Jørgensen K, Foulis AK, Richardson SJ, Morgan NG. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362–9.
Mallol C, et al. AAV-mediated pancreatic overexpression of Igf1 counteracts progression to autoimmune diabetes in mice. Mol Metab. 2017;6(7):664–80.
Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7(1):31–4.
Parikh A, Stephan A-F, Tzanakakis ES. Regenerating proteins and their expression, regulation, and signaling. Biomol Concepts. 2012;3(1):57–70.
Chen R, Meseck ML, Woo SL. Auto-regulated hepatic insulin gene expression in type 1 diabetic rats. Mol Ther. 2001;3(4):584–90.
Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000;290(5498):1959–62.
Jaen ML, et al. Long-term efficacy and safety of insulin and glucokinase gene therapy for diabetes: 8-year follow-up in dogs. Mol Ther Methods Clin Dev. 2017;6:1–7.
Ramshur EB, Rull TR, Wice BM. Novel insulin/GIP co-producing cell lines provide unexpected insights into Gut K-cell function in vivo. J Cell Physiol. 2002;192(3):339–50.
Ren B, O’Brien BA, Swan MA, Koina ME, Nassif N, Wei MQ, Simpson AM. Long-term correction of diabetes in rats after lentiviral hepatic insulin gene therapy. Diabetologia. 2007;50(9):1910–20.
Touchefeu Y, Harrington KJ, Galmiche JP, Vassaux G. Review article: gene therapy, recent developments and future prospects in gastrointestinal oncology. Aliment Pharmacol Ther. 2010;32(8):953–68.
Gan SU, Fu Z, Sia KC, Kon OL, Calne R, Lee KO. Development of a liver-specific Tet-off AAV8 vector for improved safety of insulin gene therapy for diabetes. J Gene Med. 2019;21(1):3067.
Lin Y, Sun Z. Antiaging Gene klotho attenuates pancreatic β-Cell apoptosis in type 1 diabetes. Diabetes. 2015;64(12):4298–311.
Lim K, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015;100(10):E1308–18.
Prud’homme GJ, Glinka Y, Kurt M, Liu W, Wang Q. The anti-aging protein Klotho is induced by GABA therapy and exerts protective and stimulatory effects on pancreatic beta cells. Biochem Biophys Res Commun. 2017;493(4):1542–7.
Flotyńska J, Uruska A, Araszkiewicz A, Zozulińska-Ziółkiewicz D. Klotho protein function among patients with type 1 diabetes. Endokrynol Pol. 2018;69(6):696–704.
Berezin AE, Berezin AA. Impaired function of fibroblast growth factor 23 / Klotho protein axis in prediabetes and diabetes mellitus: promising predictor of cardiovascular risk. Diabetes Metabol Syndr. 2019;13(4):2549–56.
Xie A, et al. Anti-TCRbeta mAb in combination with neurogenin3 gene therapy reverses established overt type 1 diabetes in female NOD mice. Endocrinology. 2017;158(10):3140–51.
Cox AR, et al. Resolving discrepant findings on ANGPTL8 in β-cell proliferation: a collaborative approach to resolving the betatrophin controversy. PLoS ONE. 2016;11(7):159276.
Yi P, Park J-S, Melton DA. Retraction notice to: betatrophin: A hormone that controls pancreatic β cell proliferation. Cell. 2017;168(1–2):326.
Gusarova V, et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell. 2014;159(3):691–6.
Cox AR, Lam CJ, Bonnyman CW, Chavez J, Rios JS, Kushner JA. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase beta cell proliferation in mice. Diabetologia. 2015;58(7):1523–31.
Chen J, Chen S, Huang P, Meng X-L, Clayton S, Shen J-S, Grayburn PA. In vivo targeted delivery of ANGPTL8 gene for beta cell regeneration in rats. Diabetologia. 2015;58(5):1036–44.
Chen S, Shimoda M, Wang M-Y, Ding J, Noguchi H, Matsumoto S, Grayburn PA. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther. 2010;17(11):1411–20.
Chen Y, et al. CTLA-4 +49 G/A, a functional T1D risk SNP, affects CTLA-4 level in Treg subsets and IA-2A positivity, but not beta-cell function. Sci Rep. 2018;8(1):10074.
Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–11.
Mourich DV, Oda SK, Schnell FJ, Crumley SL, Hauck LL, Moentenich CA, Marshall NB, Hinrichs DJ, Iversen PL. Alternative splice forms of CTLA-4 induced by antisense mediated splice-switching influences autoimmune diabetes susceptibility in NOD mice. Nucleic Acid Ther. 2014;24(2):114–26.
Shahryari A, Saghaeian Jazi M, Mohammadi S, Razavi Nikoo H, Nazari Z, Hosseini ES, Burtscher I, Mowla SJ, Lickert H. Development and clinical translation of approved gene therapy products for genetic disorders. Front Genetics. 2019;10:868.
Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82–83:1–11.
Lee JB, Lee JE, Park JH, Kim SJ, Kim MK, Roh SI, Yoon HS. Establishment and maintenance of human embryonic stem cell lines on human feeder cells derived from uterine endometrium under serum-free condition1. Biol Reprod. 2005;72(1):42–9.
Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121.
Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1(2):495–507.
Cai J, Yu C, Liu Y, Chen S, Guo Y, Yong J, Lu W, Ding M, Deng H. Generation of homogeneous PDX1(+) pancreatic progenitors from human ES cell-derived endoderm cells. J Mol Cell Biol. 2010;2(1):50–60.
Sui L, et al. beta-Cell replacement in mice using human type 1 diabetes nuclear transfer embryonic stem cells. Diabetes. 2018;67(1):26–35.
Qadir MMF, et al. A double fail-safe approach to prevent tumorigenesis and select pancreatic β cells from human embryonic stem cells. Stem Cell Reports. 2019;12(3):611–23.
Kotini AG, de Stanchina E, Themeli M, Sadelain M, Papapetrou EP. Escape mutations, ganciclovir resistance, and teratoma formation in human iPSCs expressing an HSVtk suicide gene. Molecular Therapy. 2016;5:284.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):7–1920.
Millman JR, Pagliuca FW. Autologous pluripotent stem cell-derived β-like cells for diabetes cellular therapy. Diabetes. 2017;66(5):1111.
Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, Millman JR. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Reports. 2019;12(2):351–65.
Voltarelli JC, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568–76.
Couri CE, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573–9.
Penaforte-Saboia JG, et al. Microvascular complications in type 1 diabetes: a comparative analysis of patients treated with autologous nonmyeloablative hematopoietic stem-cell transplantation and conventional medical therapy. Front Endocrinol (Lausanne). 2017;8:331.
Bhatwadekar AD, et al. Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: Implications for bone marrow rejuvenation. Vision Res. 2017;139:211–20.
Ye L, Li L, Wan B, Yang M, Hong J, Gu W, Wang W, Ning G. Immune response after autologous hematopoietic stem cell transplantation in type 1 diabetes mellitus. Stem Cell Res Therapy. 2017;8(1):90.
Xiang H, et al. Residual β-cell function predicts clinical response after autologous hematopoietic stem cell transplantation. Stem Cells Transl Med. 2016;5(5):651–7.
Snarski E, et al. Immunoablation and autologous hematopoietic stem cell transplantation in the treatment of new-onset type 1 diabetes mellitus: long-term observations. Bone Marrow Transplant. 2016;51(3):398–402.
Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012;71(2):439–44.
Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Investig. 2003;111(6):843–50.
Xie Q-P, Huang H, Xu B, Dong X, Gao S-L, Zhang B, Wu Y-L. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation. 2009;77(5):483–91.
Milanesi A, Lee J-W, Li Z, Da Sacco S, Villani V, Cervantes V, Perin L, Yu JS. β-Cell regeneration mediated by human bone marrow mesenchymal stem cells. PLoS ONE. 2012;7(8):42177.
Ghodsi M, Heshmat R, Amoli M, Keshtkar AA, Arjmand B, Aghayan H, Hosseini P, Sharifi AM, Larijani B. The effect of fetal liver-derived cell suspension allotransplantation on patients with diabetes: first year of follow-up. Acta Med Iran. 2012;50(8):541–6.
PubMed Google Scholar
Zhang J, Mao R, Wang X, Liu K, Geng Q, Yu Y, Li Y, Qi J. Targeted induction of bone marrow mesenchymal stem cells to have effectiveness on diabetic pancreatic restoration. Vitro Cell Dev Biol. 2019;55(6):453–61.
Qu-Petersen Z, et al. Identification of a novel population of muscle stem cells in mice. Potent Muscle Regener. 2002;157(5):851–64.
CAS Google Scholar
Lan KC, Wang CC, Yen YP, Yang RS, Liu SH, Chan DC. Islet-like clusters derived from skeletal muscle-derived stem/progenitor cells for autologous transplantation to control type 1 diabetes in mice. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S328-s335.
Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63(11):1886–92.
Amer MG, Embaby AS, Karam RA, Amer MG. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene. 2018;654:87–94.
Ikemoto T, Feng R, Iwahashi SI, Yamada S, Saito Y, Morine Y, Imura S, Matsuhisa M, Shimada M. In vitro and in vivo effects of insulin-producing cells generated by xeno-antigen free 3D culture with RCP piece. Sci Rep. 2019;9(1):10759.
Fang Q, Zhai M, Wu S, Hu X, Hua Z, Sun H, Guo J, Zhang W, Wang Z. Adipocyte-derived stem cell-based gene therapy upon adipogenic differentiation on microcarriers attenuates type 1 diabetes in mice. Stem Cell Res Ther. 2019;10(1):36.
Dessels C, Alessandrini M, Pepper MS. Factors influencing the umbilical cord blood stem cell industry: an evolving treatment landscape. Stem Cells Transl Med. 2018;7(9):643–50.
Kim Y-J, Broxmeyer HE. Immune regulatory cells in umbilical cord blood and their potential roles in transplantation tolerance. Crit Rev Oncol Hematol. 2011;79(2):112–26.
Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286.
Prabakar KR, Domínguez-Bendala J, Molano RD, Pileggi A, Villate S, Ricordi C, Inverardi L. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell Transplant. 2012;21(6):1321–39.
Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS ONE. 2009;4(1):4226.
Zhao Y, et al. Reversal of type 1 diabetes via islet β cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10(1):1–11.
Cai J, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149–57.
Haller MJ, Viener H-L, Wasserfall C, Brusko T, Atkinson MA, Schatz DA. Autologous umbilical cord blood infusion for type 1 diabetes. Exp Hematol. 2008;36(6):710–5.
Haller MJ, et al. Autologous umbilical cord blood infusion followed by oral docosahexaenoic acid and vitamin D supplementation for C-peptide preservation in children with Type 1 diabetes. Biol Blood Marrow Transplant. 2013;19(7):1126–9.
Giannopoulou EZ, et al. Effect of a single autologous cord blood infusion on beta-cell and immune function in children with new onset type 1 diabetes: a non-randomized, controlled trial. Pediatr Diabetes. 2014;15(2):100–9.
Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181(10):7350–5.
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem cells. 2004;22(7):1330–7.
Kalaszczynska I, Ferdyn K. Wharton’s Jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. BioMed Res Int. 2015;2015:430847.
Hu J, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocrine J. 2012;5:89.
Li L, Lu J, Shen S, Jia X, Zhu D. Wharton’s jelly-derived mesenchymal stem cell therapy to improve β-cell function in patients with type 1 diabetes and ketoacidosis: a single-centre, single-group, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2016;4:S17.
Hong-Wu Wang PN. Han-Hua Yang, Li-Chun Xie, Li-Min Lin, Xiu-Lan Lai, Tian-You Wang, Lian Ma, Partially repair damaged Islets of diabetic rat model via insulin-producing cells differentiated from human umbilical cord mesenchymal stem cells infusion. Int J Clin Exp Med. 2018;11(5):4520–9.
Tsai P-J, et al. Undifferentiated Wharton’s jelly mesenchymal stem cell transplantation induces insulin-producing cell differentiation and suppression of T-cell-mediated autoimmunity in nonobese diabetic mice. Cell Transplant. 2015;24(8):1555–70.
Carlsson P-O, Svahn M. Wharton’s jelly derived allogeneic mesenchymal stromal cells for treatment of type 1 diabetes: Study protocol for a double-blinded, randomized, parallel, placebo-controlled trial. Clin Trials Degener Dis. 2018;3(2):32–7.
El-Demerdash RF, Hammad LN, Kamal MM, El Mesallamy HO. A comparison of Wharton’s jelly and cord blood as a source of mesenchymal stem cells for diabetes cell therapy. Regen Med. 2015;10(7):841–55.
Som C, Venkataramana NK. Evaluation of efficacy and regenerative potential of Wharton’s jelly and bone marrow derived mesenchymal stem cells in diabetic rats. J Pre-Clin Clin Res. 2018;12(1):30–5.
Gray A, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK279012/ .
Scott SN, Anderson L, Morton JP, Wagenmakers AJM, Riddell MC. Carbohydrate restriction in type 1 diabetes: a realistic therapy for improved glycaemic control and athletic performance? Nutrients. 2019;11(5):1022.
Laurenzi A, et al. Effects of carbohydrate counting on glucose control and quality of life over 24 weeks in adult patients with type 1 diabetes on continuous subcutaneous insulin infusion: a randomized, prospective clinical trial (GIOCAR). Diabetes Care. 2011;34(4):823–7.
Evert AB, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731.
Rossi MC, et al. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care. 2010;33(1):109–15.
Kattelmann KK, Conti K, Ren C. The medicine wheel nutrition intervention: a diabetes education study with the Cheyenne River Sioux Tribe. J Am Diet Assoc. 2010;110(5):S44–51.
Cavanaugh K, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737–46.
Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–97.
Quesada I, Tudurí E, Ripoll C, Nadal A. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199(1):5–19.
Wieczorek G, Pospischil A, Perentes E. A comparative immunohistochemical study of pancreatic islets inlaboratory animals (rats, dogs, minipigs, nonhuman primates). Exp Toxicol Pathol. 1998;50(3):151–72.
Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci. 2006;103(7):2334–9.
Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–45.
Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15(1):620.
Chen Y-G, Mathews CE, Driver JP. The role of NOD mice in type 1 diabetes research: lessons from the past and recommendations for the future. Front Endocrinol. 2018;9:51.
Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60(8):1370–81.
Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Prim. 2017;3(1):1–17.
Shanks N, Greek R, Greek J. Are animal models predictive for humans? PEHM. 2009;4:2–2.
PubMed PubMed Central Google Scholar
Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult β-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology. 2009;150(4):1627–35.
Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia. 2006;49(8):1838–44.
Sims EK, et al. Proinsulin Secretion Is a Persistent Feature of Type 1 Diabetes. Diabetes Care. 2019;42(2):258.
Wasserfall C, et al. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab. 2017;26(3):568-575.e3.
Rehan M. Epigenetics and diabetes mellitus. Egyp J Int Med. 2016;28(2):39–51.
Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011;91(1):94–100.
Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, Ricordi C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep. 2017;7(1):5998.
Jerram ST, Dang MN, Leslie RD. The role of epigenetics in type 1 diabetes. Curr DiabRep. 2017;17(10):89–89.
Chakravarthy H, et al. Converting adult pancreatic islet alpha cells into beta cells by targeting Both Dnmt1 and Arx. Cell Metab. 2017;25(3):622–34.
Fontcuberta-PiSunyer M, Cervantes S, Miquel E, Mora-Castilla S, Laurent LC, Raya A, Gomis R, Gasa R. Modulation of the endocrine transcriptional program by targeting histone modifiers of the H3K27me3 mark. Biochim Biophys Acta Gene Regul Mech. 2018;1861(5):473–80.
Akil A-SA-S, et al. Reading between the (Genetic) lines: How epigenetics is unlocking novel therapies for type 1 diabetes. Cells 2020;9(11):2403.
Beger RD, et al. Metabolomics enables precision medicine: “A White Paper, Community Perspective.” Metabolomics. 2016;12(9):149.
Cooper-Dehoff RM, et al. Is diabetes mellitus-linked amino acid signature associated with beta-blocker-induced impaired fasting glucose? Circ Cardiovasc Genet. 2014;7(2):199–205.
Jobin C. Precision medicine using microbiota. Science. 2018;359(6371):32–4.
Bart O. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. 2019;7(1):65–74.
Download references
Acknowledgements
We wish to thank Lucy Robinson of Insight Editing London for assistance with editing support and critical reading of the manuscript prior to submission.
This research was funded by Sidra Medicine through its Precision Medicine Program Grant—SDR#400149, Doha, Qatar.
Author information
Authors and affiliations.
Department of Human Genetics-Precision Medicine Program, Sidra Medicine, P.O. Box 26999, Doha, Qatar
Ammira Al-Shabeeb Akil, Esraa Yassin, Aljazi Al-Maraghi, Elbay Aliyev, Khulod Al-Malki & Khalid A. Fakhro
Department of Genetic Medicine, Weill Cornell Medicine, P.O. Box 24144, Doha, Qatar
Khalid A. Fakhro
College of Health and Life Sciences, Hamad Bin Khalifa University, P.O. Box 34110, Doha, Qatar
You can also search for this author in PubMed Google Scholar
Contributions
Original draft preparation, ASA; review and editing, AAM; KAM; EY, EA and KF; visualization, ASA; supervision, ASA; KF; project administration, ASA; funding acquisition, ASA. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ammira Al-Shabeeb Akil .
Ethics declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Akil, A.AS., Yassin, E., Al-Maraghi, A. et al. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med 19 , 137 (2021). https://doi.org/10.1186/s12967-021-02778-6
Download citation
Received : 18 December 2020
Accepted : 08 March 2021
Published : 01 April 2021
DOI : https://doi.org/10.1186/s12967-021-02778-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Type 1 diabetes
- Autoimmunity
- Personalized medicine
- Personalized treatment
- Genomic Risk Score
- Insulin therapy
- Gene polymorphism
- Pancreatic β cells
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Type 1 diabetes: A predictable disease
Kimber m simmons, aaron w michels.
- Author information
- Article notes
- Copyright and License information
Author contributions: Simmons KS and Michels AW contributed to this paper.
Correspondence to: Aaron W Michels, MD, Barbara Davis Center for Childhood Diabetes, University of Colorado Denver, Mail Stop A140, 1775 Aurora Court, Aurora, CO 80045, United States. [email protected]
Telephone: +1-303-7241923 Fax: +1-303-7246784
Received 2014 Sep 11; Revised 2014 Nov 26; Accepted 2015 Jan 9; Issue date 2015 Apr 15.
Type 1 diabetes (T1D) is an autoimmune disease characterized by loss of insulin producing beta cells and reliance on exogenous insulin for survival. T1D is one of the most common chronic diseases in childhood and the incidence is increasing, especially in children less than 5 years of age. In individuals with a genetic predisposition, an unidentified trigger initiates an abnormal immune response and the development of islet autoantibodies directed against proteins in insulin producing beta cells. There are currently four biochemical islet autoantibodies measured in the serum directed against insulin, glutamic decarboxylase, islet antigen 2, and zinc transporter 8. Development of islet autoantibodies occurs before clinical diagnosis of T1D, making T1D a predictable disease in an individual with 2 or more autoantibodies. Screening for islet autoantibodies is still predominantly done through research studies, but efforts are underway to screen the general population. The benefits of screening for islet autoantibodies include decreasing the incidence of diabetic ketoacidosis that can be life threatening, initiating insulin therapy sooner in the disease process, and evaluating safe and specific therapies in large randomized clinical intervention trials to delay or prevent progression to diabetes onset.
Keywords: Autoimmunity, Autoantibodies, Diabetes prevention, Screening, Type 1 diabetes
Core tip: Type 1 diabetes (T1D), the immune mediated form of diabetes, is now a predictable disease with the measurement of islet autoantibodies. The presence of two or more antibodies defines preclinical disease as nearly everyone with multiple antibodies progresses to clinical diabetes. With improved platforms to measure islet autoantibodies, screening the general population is now a goal. Early identification of preclinical diabetes allows for less diabetic ketoacidosis, early initiation of insulin therapy, and the potential to delay or prevent diabetes onset. Clinical trials using safe and specific therapies to block disease specific immune cells are underway in T1D.
INTRODUCTION
Type 1 diabetes (T1D) is a chronic disease caused by immune-mediated destruction of insulin producing beta cells in the pancreas[ 1 ]. The destruction of beta cells results in insulin insufficiency, and patients develop life-threatening hyperglycemia that clinically manifests with weight loss, polyuria, and polydipsia. The majority of patients who develop T1D have high-risk human leukocyte antigen ( HLA ) genes. Islet autoantibodies can be measured in the serum of these high-risk individuals years before the onset of any clinical symptoms, making T1D a predictable disease. Multiple prevention trials in patients with high-risk HLA genes or in patients who have measureable autoantibodies have been completed. To date, no trial has prevented the onset of T1D, but data indicates that the disease process may be delayed by administering oral insulin to induce insulin specific regulatory T-cells in the gut, resulting in decreased inflammation in the pancreas. This review summarizes the epidemiology, risk factors and pathogenesis of T1D. The review also examines the goal of screening the general population for T1D risk and preventing disease onset in individuals with preclinical disease.
EPIDEMIOLOGY
T1D is one of the most common chronic diseases in childhood and is diagnosed at an increasing rate in adults. The incidence rate varies significantly by geographical region. Sweden, Finland, Norway, United Kingdom, and Sardinia have the highest incidence of T1D at an age-adjusted rate of > 20/100000 patient years. For comparison, the United States has an incidence rate of 17.8/100000 patient years in a predominantly Caucasian population. China and South America have the lowest incidence of T1D, reported as < 1/100000 patient years[ 2 - 5 ]. The rate of T1D diagnosis is increasing in most countries, with rates dramatically increasing in children less than 5 years of age[ 6 ]. The annual incidence of T1D is increasing globally by 2.3% per year and is estimated to be increasing by 2.7%-2.8% in non-Hispanic white youth in the United States[ 7 ]. Large registries in both Europe and the United States show that the incidence of T1D peaks between 5 to 7 years of age and again when children enter puberty[ 8 ]. Unlike most autoimmune diseases, T1D is more common in males than females. The risk of T1D development in the general population is 1:300[ 9 ]. In children who have a genetically related sibling, the risk is increased to 1:7 and is greatest in children under 5 years of age[ 10 , 11 ]. Offspring of mothers with T1D carry approximately 3% risk and offspring of fathers with T1D carry approximately 5% risk[ 12 ]. Genetics confer risk for development of T1D, as does seasonal variation and birth month suggesting an environmental influence on disease pathogenesis. Children born in the spring tend to be at a greater risk for developing T1D, while diagnosis is increased during climatically cold seasons[ 13 - 16 ]. This is an epidemiological association that requires further investigation.
RISK FACTORS
T1D is a polygenic disorder with many genes contributing varying amounts of genetic risk for disease development. The genes conferring risk for diabetes are generally classified as HLA and non-HLA genes. Large genome wide association studies show that over 40 genes increase susceptibility to T1D[ 17 , 18 ]. The major determinant of genetic susceptibility to T1D, contributing greater than 50% of the genetic risk, is conferred by genes in the HLA complex located on chromosome 6[ 9 ]. The HLA complex is divided into 3 regions: classes I, II, and III. Alleles of the class II genes, DQ and DR (and to a lesser extent DP), are the most important determinants of T1D. These class II molecules are expressed on antigen-presenting cells (macrophages, dendritic cells, and B cells) and present antigens to CD4 T lymphocytes. DQ and DR genes are in close linkage disequilibrium on chromosome 6 with specific DQ and DR genes inherited together. The presence of the DR4/DQ8 haplotype increases the odds ratio for T1D development to approximately 11, indicating an individual with this haplotype is 11 times more likely to develop T1D than those without. Approximately 90% of all individuals with T1D have either or both the DR4/DQ8 or DR3/DQ2 haplotypes. Interestingly, HLA genes also confer protection from T1D development. Individuals who have the specific DQ6 allele (DQB1*06:02) are dominantly protected from T1D, with an odds ratio of 0.03 for disease development[ 19 ].
Of the non-HLA genes, insulin and protein tyrosine phosphatase non-receptor type 22 (PTPN22) confer risk for T1D development but to lesser degrees than HLA genes[ 20 ]. Similar to HLA class II genes, insulin gene polymorphisms can confer both susceptibility to and protection from T1D development. At the 5’ end of the insulin gene, there are variable numbers of tandem repeats. Having more repeats correlates to more insulin message being expressed in the thymus. The thymus responds by developing central tolerance to insulin. In individuals with fewer repeats, autoreactive T-cells can persist, and the risk for T1D development is increased[ 21 ]. PTPN22 helps regulate antigen receptor signaling and T cell activation, and a single nucleotide polymorphism (arginine to tryptophan at position 620) has been associated with a number of autoimmune disorders including T1D. A gain of function polymorphism decreases T cell receptor signaling which confers diabetes risk. It is unknown why decreased T cell activation leads to T1D risk, but it can be hypothesized that deficient negative selection of thymic cells may be involved[ 22 , 23 ].
Environment
Genetics alone does not lead to T1D; the environment also plays a pivotal role. This is evidenced by the fact that not all individuals with high-risk genes develop T1D. In fact, the majority of individuals with high-risk HLA class II genes (DR4/DQ8 and DR3/DQ2) do not develop T1D. There are likely one or more environmental factors that trigger and perpetuate the autoimmune disease process prior to hyperglycemia and a clinical diagnosis of hyperglycemia and T1D. Large natural history studies indicate that the development of islet autoantibodies (the first laboratory evidence of beta cell autoimmunity) in high-risk individuals often occurs between 9 mo and 2 years of age[ 24 ]. This suggests that an environmental trigger is present early in life, possibly in utero .
One of the most extensively evaluated environmental triggers is viral infection. Many viruses are implicated in the development of T1D including enteroviruses such as coxsackie B virus, cytomegalovirus, congenital rubella syndrome, and rotavirus[ 25 - 32 ]. Enterovirus is the leading candidate for contributing to T1D development. Epidemiologic studies in Finland show that the development of beta cell autoimmunity parallels the seasonal pattern of enterovirus infection and clinical symptoms of enteroviral infection[ 33 , 34 ]. Enterovirus infection was strongly associated with the development of autoantibodies in the Diabetes Autoimmunity Study in the Young (DAISY) cohort[ 35 ]. Laboratory evidence of enterovirus infection is reproducibly present in individuals with new onset T1D, pregnant women whose children develop T1D, and donor pancreata of individuals with T1D[ 36 , 37 ]. The exact mechanism of how viruses induce autoimmunity is not clear. The molecular mimicry hypothesis proposes that because the P2-C protein sequence of enterovirus is similar to glutamic decarboxylase (GAD), which is expressed in islet cells, the immune system erroneously targets destruction of beta cells[ 38 ]. The other leading hypothesis is that viral infection activates autoreactive T cells. As evidence, Cytomegalovirus B4 has tropism for pancreatic tissue and infection results in release of beta cell antigens that are phagocytized by macrophages and presented to autoreactive T cells[ 39 ].
Another potential environmental influence relates to the north-south division of diabetes development in the world, with a higher incidence of T1D in northern climates compared to southern. The north-south hypothesis implicates that a lack of vitamin A and/or D exposure early in life predisposes individuals to the development of autoimmune diseases including T1D. Offspring of mothers supplemented with vitamin D during pregnancy and young children supplemented with vitamin D have shown a reduced risk of T1D development that may be dose responsive[ 40 , 41 ]. However, an analysis from the DAISY Study found that vitamin D intake and 25(OH) vitamin D levels throughout childhood were not associated with the development of islet autoantibodies or T1D development[ 42 ].
Early introduction of cow’s milk and gluten have also been extensively studied. The introduction of gluten into an infant’s diet prior to three months and after 7 mo has been associated with increased autoantibody development[ 24 , 43 ]. Some studies also indicate that breastfeeding or using elemental formula may be protective against T1D. Other environmental factors that continue to be explored include nitrosamine compounds, maternal age, pre-eclampsia, and childhood obesity. There is no evidence to suggest that vaccines increase the risk of T1D development[ 44 ]. To date, there are no causal environmental factors that trigger the development of islet autoantibodies or increase the risk of progression to clinical T1D development. However, there is a large international prospective longitudinal study, The Environmental Determinants of Diabetes in the Young, currently underway to evaluate potential environmental factors in T1D[ 45 ].
NATURAL HISTORY
Three decades ago, it was hypothesized that T1D is a chronic autoimmune disorder that develops in stages, and the model remains valid today (Figure 1 ). In genetically predisposed individuals (those with DR4/DQ8 and/or DR3/DQ2 haplotypes) there is an environmental trigger that leads to a break in immunologic tolerance and loss of beta cell mass. Over a period of time, usually years, there is autoimmune destruction of insulin producing beta cells that is marked by the presence of serum islet autoantibodies (Figure 2 ). As the process continues, very likely in a relapsing and remitting manner, there is a loss of glucose stimulated insulin release, and eventually insulin deficiency such that overt hyperglycemia results and clinical T1D is diagnosed[ 4 ].
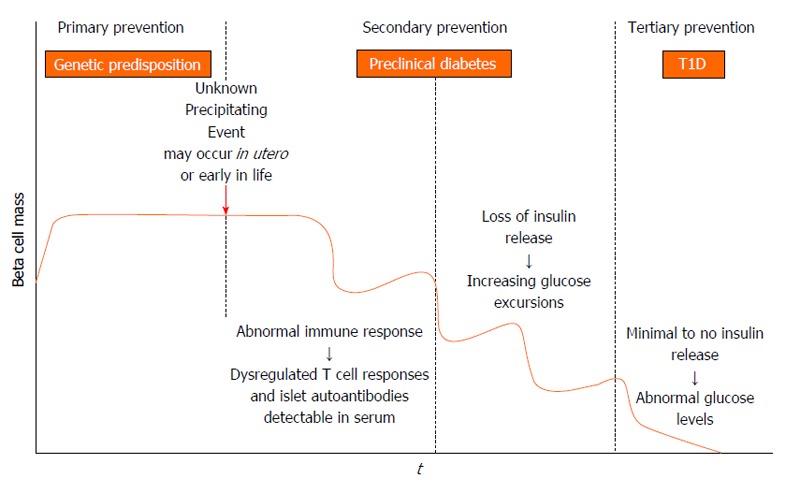
Stages in the development of type 1 diabetes adapted from the initial model proposed by George Eisenbarth. In genetically at risk individuals an unknown trigger, presumably environmental, initiates an autoimmune response that results in loss of beta cell mass. Before metabolic disturbances occur, islet autoantibodies (insulin, glutamic decarboxylase, islet antigen 2, zinc transporter 8) are measureable in serum. As beta cell mass decreases, potentially in a relapsing-remitting manner, there is loss of endogenous insulin release and ensuing hyperglycemia. Within this model, there are opportunities for type 1 diabetes (T1D) prevention in genotypically high risk individuals (primary prevention) and in autoantibody positive individuals (secondary prevention). Interventions to preserve remaining beta cell mass at diagnosis are also possible (tertiary prevention).
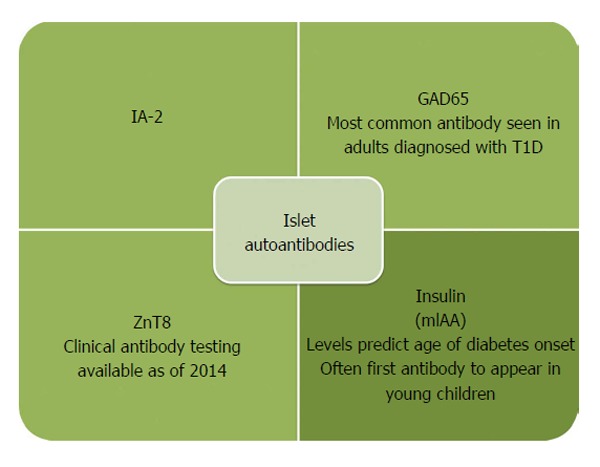
Insulin autoantibodies are often the first antibody to develop in young children. In contrast, adults most often are GAD65 and IA-2 autoantibody positive at diagnosis. The ZnT8 antibody is the most recently identified autoantibody with commercial testing now available. T1D: Type 1 diabetes; GAD65: Glutamic decarboxylase; IA-2: Islet antigen 2; ZnT8: Zinc transporter 8.
How an inciting event leads to an aberrant immune response is not completely understood. Most hypotheses focus on immunologic abnormalities in antigen presentation by HLA molecules to T cells in the thymus and peripheral lymph organs. T cells are educated in the thymus to self-antigens, such as insulin, and if there are dysregulated immune processes, self-reactive T cells can escape central tolerance and exist in the periphery[ 46 , 47 ]. Once these cells encounter their target antigen or peptide in peripheral lymph organs, they become activated to target beta cells. Other hypotheses focus on environmental triggers leading to immune activation and targeting of beta cells. The molecular mimicry theory proposes that a viral or bacterial protein shares amino acid sequence homology with beta cells and induces immune system activation through targeting beta cell antigen that is molecularly similar to a foreign antigen[ 38 ]. Finally an infectious triggering event may allow beta cells to become more sensitive to cytokine and free radical induced inflammation[ 48 ].
Recently the network for pancreatic organ donors has been established to study the pancreata of deceased donors with islet autoantibodies (preclinical disease) or established T1D[ 49 ]. The goal is to understand mechanisms of disease pathogenesis and interactions between beta cells and the immune system[ 50 ]. What we have gleaned from the initial efforts is that islet infiltrates (insulitis) are present in a lobular pattern in the pancreas, and there is a predominance of CD8 and CD4 T cells, B-lymphocytes, and macrophages[ 51 , 52 ]. Pancreata from established T1D patients also show an overall decrease in weight compared to age matched controls, potentially related to atrophy of the exocrine pancreas with the loss of beta cells[ 52 ]. The first serological evidence of an autoimmune response to beta cells is the appearance of autoantibodies to insulin (IAA), GAD, islet antigen 2, and zinc transporter 8[ 53 ]. Placental antibodies are no longer present after approximately 6 mo, so any antibodies in serum after that time reflect endogenous antibodies. If an individual develops two or more of these antibodies, they will eventually progress to clinical onset of T1D[ 54 ]. Approximately 90% of individuals have two or more islet cell autoantibodies at diagnosis, and it is likely that the remaining 10% of individuals (islet autoantibody negative) have autoantibodies against antigens that have yet to be discovered. In children, IAA is usually the first antibody to develop, and the progression to T1D is 100% in children with a persistently high level of IAA[ 55 , 56 ]. This is in contrast to adults who tend to have higher levels of GAD at diagnosis. Islet autoantibodies can be easily measured in the serum, with the gold standard method for detecting antibodies being fluid phase radioimmunoassays (RIA)[ 57 ]. More recently, islet autoantibodies are now able to be measured from smaller volumes of serum and without the use of radioactivity using electrochemiluminescense as a detection method while maintaining similar sensitivity and specificity to RIA[ 58 , 59 ]. The rate at which individuals with positive islet autoantibodies progress to clinical T1D is dependent upon the age of appearance, insulin autoantibody level, and the number of autoantibodies present[ 55 ]. Hemoglobin A1c rises 1 to 1.5 years prior to diagnosis. Therefore, reduced insulin secretion and resultant hyperglycemia occur before T1D is clinically diagnosed[ 60 , 61 ]. Once T1D is clinically diagnosed, individuals must commit to lifelong blood glucose monitoring and intensive insulin administration via multiple daily injections or an insulin pump to achieve good glycemic control. With improved diabetes management, the risk for long-term complications such as renal failure, myocardial infarctions, stroke, and lower extremity amputations has decreased over the last two decades[ 62 ]. However despite the decreasing prevalence of complications in diabetes, the need still exists to understand the underlying pathogenesis of complications such as diabetic cardiomyopathy and novel approaches for treating complications such as neovascularization in diabetic foot disease[ 63 , 64 ].
SCREENING AND PREVENTION
The American Diabetes Association recently adapted their guidelines to recommend screening for islet autoantibodies in high-risk individuals[ 65 ]. Highly sensitive serological assays are not widely available, and all screening is recommended to be done in the setting of a clinical research study. To date, general population screening has been done through large clinical trial networks such as the National Institutes of Health sponsored TrialNet, which enroll and screen first or second degree family members of individuals with T1D. By identifying individuals with positive islet autoantibodies, the rate of diabetic ketoacidosis (DKA) at diagnosis is reduced[ 66 ]. Preventing DKA is important as altered mental status, coma, and even death can occur[ 67 ]. In fact, DKA is the most common cause of death in children with T1D[ 68 ]. Without screening, DKA at diagnosis is relatively common[ 69 ]. In the EURODIAB study, 42% of children presented in DKA (pH < 7.3) at the time of diagnosis with T1D[ 3 ]. By identifying individuals with positive autoantibodies, insulin therapy can be initiated early, and these children can enroll in studies aimed at preserving beta cell mass. In adults, maintaining endogenous insulin secretion reduces hemoglobin A1C, reduces the risk of severe hypoglycemia, decreases reliance on exogenous insulin, and decreases the rate of long-term complications[ 70 - 77 ]. In children, there has been very little data collected regarding residual beta cell mass beyond the first year after diagnosis[ 78 - 82 ]. A case-control study did show that children without severe hypoglycemia had increased residual beta cell mass compared to those children with severe hypoglycemia[ 83 ]. An effective method of preserving beta cell mass is not yet available, and the benefit of increased residual beta cell mass in children remains to be confirmed.
According to the World Health Organization’s principles of early disease detection, T1D is a condition that meets criteria for the establishment of a screening program. These principles include the condition is an important health problem, there is a recognizable latent stage of the disease, the natural history of the disease is understood, there is an adequate and accepted laboratory screening test, providers agree on who should receive treatment and there is a treatment available, there are adequate resources for diagnosis and treatment, and the cost of overall medical care would not increase[ 84 ]. Islet autoantibodies can be reliably measured in serum, with each antibody assay having a specificity of 99% when measured by radioimmunoassay in tertiary referral centers such as the Barbara Davis Center for Diabetes. The sensitivity for each autoantibody assay ranges from 70%-80%. We view these radioimmunoassays as a confirmatory test for T1D. A desired screening test needs to be reliable with high sensitivity, cost effective, and technically feasible, likely as a multiplex assay in which all four autoantibodies are measured in a single well of an assay plate. Currently, to measure islet autoantibodies a blood draw is required with subsequent shipping of venous or capillary blood samples to a reference laboratory. This is not feasible for population wide screening due to technical requirements of sample collection and high cost. Screening large populations of infants for metabolic diseases and other congenital disorders has been successfully done using dried blood spots[ 85 ]. To establish an accepted screening program for T1D, the sensitivity and specificity of islet autoantibodies, specifically insulin autoantibody, needs to be established using a feasible collection method such as dried blood spots on filter paper, which would be a simplified collection method and more cost effective. Overall, T1D would not be over diagnosed with general population screening as diagnosis of the disorder requires both the presence of islet autoantibodies and metabolic abnormalities.
Ideally, individuals who screen positive for islet autoantibodies can be offered a treatment to prevent or delay the progression to T1D. Many secondary prevention trials have been completed with more currently underway[ 86 ]. As population based screening may be feasible in the near future, it is important to continue secondary prevention trials with the goal of delaying or preventing progression to T1D in islet autoantibody positive individuals. Patients enrolled in clinical intervention trials benefit from close follow up by medical professionals, early diagnosis of T1D, decreased incidence of DKA, and early initiation of insulin therapy (Figure 3 ).
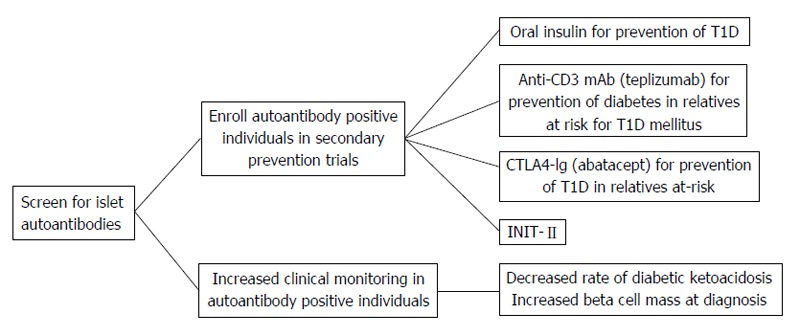
The measurement of serum islet autoantibodies has made type 1 diabetes a predictable disease. Early identification of islet autoantibody positive individuals leads to improved clinical outcomes by decreasing the risk for diabetic ketoacidosis and potentially preserving beta cell mass through clinical prevention trials. T1D: Type 1 diabetes; INIT-II: Intranasal Insulin Trial.
Secondary prevention trials
As T1D is a predictable disease with the measurement of islet autoantibodies, it logically follows that the disease should be preventable. To date, the majority of secondary prevention trials (enrolled individuals with preclinical disease) have administered different preparations of insulin to autoantibody positive individuals in an attempt to slow the progression to T1D onset[ 87 ]. The first such trial was the diabetes prevention trial-type 1 in which at risk patients were either administered subcutaneous insulin or oral insulin in randomized, double-blinded, placebo controlled trials. Oral insulin has no metabolic effect; however, orally administered insulin does encounter mucosal gut-associated lymphoid tissue. The role of this lymphoid tissue is to provide protection from orally acquired pathogens and to keep individuals from developing reactions to ingested proteins. By administering low doses of oral insulin, insulin-specific T-regulatory cells are produced which may release cytokines that inhibit the inflammatory cascade that leads to β-cell destruction[ 88 - 90 ]. Relatives of patients with T1D who were 3 to 45 years of age and had high-risk HLA genes and one or more positive autoantibodies were evaluated for abnormal glucose metabolism. Those individuals who had abnormal glucose tolerance ( n = 339) were administered 0.25 units/kg per day of Ultralente insulin twice daily and received an intravenous insulin infusion for four days at the beginning of the study and then annually. There was no effect of low-dose subcutaneous insulin on delaying the progression to T1D[ 91 ]. Participants with normal glucose tolerance received 7.5 mg/kg per day of oral insulin ( n = 372). An oral glucose tolerance test was completed every 6 mo during a 6-year follow up, and there was not a delay in progression to T1D. Of interest, a post-hoc analysis showed in participants with persistently high levels of IAA (≥ 80 nU/mL) there was a delay in disease onset of approximately five years[ 92 ]. Also, the rate of progression to T1D onset after stopping insulin was more rapid[ 93 ]. A follow-up oral insulin trial through TrialNet is currently enrolling participants in order to determine if oral insulin can delay the progression to T1D in individuals with high IAA levels (ClinicalTrials.gov Identifier: NCT00419562 ).
Another insulin intervention trial from the Belgian T1D Registry identified study participants who were insulin autoantibody positive and did not have a HLA haplotype conferring protection (DQB*0602). Study participants were given two subcutaneous injections of insulin daily for 3 years ( n = 25) or observed and prospectively followed ( n = 25). The participants who were treated with insulin and those who refused treatment or agreed to observation developed T1D at the same rate[ 94 ].
Many preclinical studies have suggested that administration of intranasal insulin may delay T1D development through mucosal tolerance, in which mucosal antigens have been shown to impact regulatory T cell development[ 95 ]. To translate these findings to humans, individuals with high-risk HLA haplotypes and one or more islet autoantibodies were enrolled in the Intranasal Insulin Trial (INIT-I) ( n = 38). This randomized, double-blinded, crossover pilot study suggested that intranasal insulin protects against the development of T1D by increasing antibody formation and decreasing T cell responsiveness[ 96 ]. The INIT-II, a randomized, double-blinded, placebo controlled trial, is now enrolling individuals to determine if intranasal insulin can delay or prevent the progression to T1D (ClinicalTrials.gov Identifier: NCT 00336674 ). However, a large study in Finland, the T1D Prediction and Prevention Trial enrolled and followed siblings of children with T1D or infants of mothers with T1D who had high-risk HLA genes for islet autoantibody development. Once two or more autoantibodies were detected ( n = 264), they were randomized to intranasal insulin ( n = 137) or placebo ( n = 127). Interim analyses showed no benefit of intranasal insulin in delaying the onset of T1D[ 97 ]. This indicates that intranasal insulin may not be effective at delaying diabetes onset at the administered dose and timing in the disease process. Potentially insulin antigen specific therapies may need to be administered earlier in the disease course to have an impact on delaying progression to T1D.
Several trials using non-antigen specific therapies including bacille calmette-guerin injections, Ketotifen (histamine antagonist), oral cyclosporine, and nicotinamide (B6) have been completed. No study has prevented or delayed T1D development[ 98 - 104 ].
Clinical trials with drugs aimed at modulating the immune response and preserving endogenous insulin secretion in patients with new-onset T1D are termed tertiary prevention trials[ 51 ]. Only recently have these drugs expanded to prevention trials in islet autoantibody positive individuals (Figure 3 ). The CTLA4-Ig antibody (Abatacept) for Prevention of Abnormal Glucose Tolerance and Diabetes in Relatives At-Risk (ClinicalTrials.gov Identifier: NCT 01773707 ) and Anti-CD3 monoclonal antibody (Teplizumab) for Prevention Of Diabetes In Relatives At Risk For T1D mellitus (ClinicalTrials.gov Identifier: NCT01030861 ) are both TrialNet studies currently enrolling participants. Abatacept is a fusion antibody that binds to antigen presenting cells and blocks co-stimulation to T cells. Anti-CD3 monoclonal antibodies bind the CD3 molecule which is present on CD8 and CD4 T cells, thereby inhibiting T cell activation[ 105 ]. Both of these drugs have shown some degree of success when used in new-onset trials[ 106 - 111 ]. DIAPREV-IT is an antigen-based treatment currently enrolling individuals who are positive for GAD and one or more additional autoantibodies (ClinicalTrials.gov Identifier: NCT 01122446 ). A GAD/alum vaccine is given at enrollment and 1 mo later. Although GAD vaccination is safe and easily administered, new-onset intervention trials have not shown long-term preservation of endogenous insulin secretion[ 108 , 112 , 113 ].
NOVEL APPROACHES TO PREVENT T1D
Currently, insulin is the only medication approved by the United States Food and Drug Administration for the treatment of T1D. Despite T1D being a predictable chronic autoimmune disorder, there are not any therapies to preserve endogenous insulin production. As mentioned above, many large clinical intervention trials have not slowed the progression or prevented disease onset. We believe T1D will be preventable and that safe and specific therapies targeting the immune system are needed. One such approach is to target the trimolecular complex, which consists of a self-reactive CD4 T cell, insulin, and HLA molecule[ 114 ]. It is well established that specific HLA alleles, namely HLA DQ8 which is present in approximately 60% of all T1D patients, confer significant disease risk. DQ8 is a molecular target for diabetes intervention by using small “drug-like” molecules to block antigen presentation, thereby inhibiting specific T cell activation. Preclinical studies have shown this to be a potential pathway for diabetes intervention[ 115 ]. This concept has been advanced from bench to bedside as a clinical trial in which methyldopa (Aldomet), a clinically well-established antihypertensive drug, is being investigated to block DQ8 antigen presentation. The phase 1b dose escalation trial is using personalized medicine as methyldopa is being administered to recent onset adult T1D patients with the presence of the DQ8 gene (ClinicalTrials.gov Identifier: NCT01883804 ). Methyldopa is orally administered, safe as it has been used clinically for the last 50 years, and currently indicated for the treatment of pregnancy induced hypertension. Furthermore, all individuals have three class II molecules (DQ, DR, and DP), and by blocking a single class II molecule, there are two others to permit normal immune system function.
Other approaches have targeted components of the insulin trimolecular complex including antibodies that specifically bind to an insulin peptide in the HLA molecule. Preclinical studies in an animal model of spontaneous autoimmune diabetes indicate that this approach can delay diabetes onset[ 116 ]. Efforts are currently being made to make a human antibody, which again is a very specific immune therapy for diabetes intervention. Finally, insulin antigen specific therapy has the potential to evolve with recent advances in the field of immunology. A peptide from the insulin B chain amino acids 9-23 (B:9-23) has been extensively studied in animal models and human T1D[ 117 , 118 ]. It is now appreciated that insulin B:9-23 is a key autoantigen in the disease process of both mice and humans, sharing an identical amino acid sequence in both species[ 119 , 120 ]. A mutated insulin B:9-23 peptide, but not the native peptide sequence, induced protective immune responses (regulatory T cells) and prevented diabetes onset in preclinical animal models[ 121 ]. With a deeper understanding of how the insulin peptide binds to HLA molecules and activates T cells, an insulin vaccine again holds promise for diabetes prevention.
In conclusion, T1D is now a predicable disease with the measurement of islet autoantibodies and prevention will naturally follow. To prevent T1D, general population screening for islet autoantibodies is needed along with a safe and specific therapy for disease intervention. The genes that confer diabetes risk are now molecular targets, and tailoring therapies to specific HLA genes is personalized medicine. The future holds promise for delaying the progression and ultimately preventing diabetes.
P- Reviewer: Ciccone MM, Faienza MF, Haidara M, Ilangumaran S, Gray SG
S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
Supported by Grants from the National Institute of Diabetes and Digestive Kidney Diseases, No. R01 DK032083, K08 DK095995; Juvenile Diabetes Research Foundation, the Children’s Diabetes Foundation, and the Brehm Coalition.
Conflict-of-interest: The authors have no conflicts-of-interest relevant to this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 11, 2014
First decision: November 14, 2014
Article in press: January 12, 2015
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS, Hamman RF, Klingensmith G, Eisenbarth GS, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Lévy-Marchal C, Patterson CC, Green A; EURODIAB ACE Study Group. Europe and Diabetes. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and Dibetes. Diabetologia. 2001;44 Suppl 3:B75–B80. doi: 10.1007/pl00002958. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Lawrence JM, Imperatore G, Dabelea D, Mayer-Davis EJ, Linder B, Saydah S, Klingensmith GJ, Dolan L, Standiford DA, Pihoker C, et al. Trends in incidence of type 1 diabetes among non-Hispanic white youth in the U.S., 2002-2009. Diabetes. 2014;63:3938–3945. doi: 10.2337/db13-1891. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Redondo MJ, Eisenbarth GS. Genetic control of autoimmunity in Type I diabetes and associated disorders. Diabetologia. 2002;45:605–622. doi: 10.1007/s00125-002-0781-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Nokoff N, Rewers M. Pathogenesis of type 1 diabetes: lessons from natural history studies of high-risk individuals. Ann N Y Acad Sci. 2013;1281:1–15. doi: 10.1111/nyas.12021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Gillespie KM, Gale EA, Bingley PJ. High familial risk and genetic susceptibility in early onset childhood diabetes. Diabetes. 2002;51:210–214. doi: 10.2337/diabetes.51.1.210. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Hämäläinen AM, Knip M. Autoimmunity and familial risk of type 1 diabetes. Curr Diab Rep. 2002;2:347–353. doi: 10.1007/s11892-002-0025-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Rothwell PM, Gutnikov SA, McKinney PA, Schober E, Ionescu-Tirgoviste C, Neu A. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. European Diabetes Study Group. BMJ. 1999;319:887–888. doi: 10.1136/bmj.319.7214.887. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Vaiserman AM, Carstensen B, Voitenko VP, Tronko MD, Kravchenko VI, Khalangot MD, Mechova LV. Seasonality of birth in children and young adults (0-29 years) with type 1 diabetes in Ukraine. Diabetologia. 2007;50:32–35. doi: 10.1007/s00125-006-0456-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Ostman J, Lönnberg G, Arnqvist HJ, Blohmé G, Bolinder J, Ekbom Schnell A, Eriksson JW, Gudbjörnsdottir S, Sundkvist G, Nyström L. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med. 2008;263:386–394. doi: 10.1111/j.1365-2796.2007.01896.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Kordonouri O, Shuga N, Lewy H, Ashkenazi I, Laron Z. Seasonality of month of birth of children and adolescents with type 1 diabetes mellitus in Berlin differs from the general population. Eur J Pediatr. 2002;161:291–292. doi: 10.1007/s00431-002-0941-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, Erlich HA. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59:1134–1148. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet. 2011;12:781–792. doi: 10.1038/nrg3069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Bradfield JP, Qu HQ, Wang K, Zhang H, Sleiman PM, Kim CE, Mentch FD, Qiu H, Glessner JT, Thomas KA, et al. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet. 2011;7:e1002293. doi: 10.1371/journal.pgen.1002293. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Steck AK, Baschal EE, Jasinski JM, Boehm BO, Bottini N, Concannon P, Julier C, Morahan G, Noble JA, Polychronakos C, et al. rs2476601 T allele (R620W) defines high-risk PTPN22 type I diabetes-associated haplotypes with preliminary evidence for an additional protective haplotype. Genes Immun. 2009;10 Suppl 1:S21–S26. doi: 10.1038/gene.2009.87. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Snell-Bergeon JK, Smith J, Dong F, Barón AE, Barriga K, Norris JM, Rewers M. Early childhood infections and the risk of islet autoimmunity: the Diabetes Autoimmunity Study in the Young (DAISY) Diabetes Care. 2012;35:2553–2558. doi: 10.2337/dc12-0423. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Hyöty H, Leinikki P, Reunanen A, Ilonen J, Surcel HM, Rilva A, Käär ML, Huupponen T, Hakulinen A, Mäkelä AL. Mumps infections in the etiology of type 1 (insulin-dependent) diabetes. Diabetes Res. 1988;9:111–116. [ PubMed ] [ Google Scholar ]
- 26. Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia. 2002;45:1353–1361. doi: 10.1007/s00125-002-0852-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison LC. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49:1319–1324. doi: 10.2337/diabetes.49.8.1319. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Honeyman MC, Stone NL, Harrison LC. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol Med. 1998;4:231–239. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Pak CY, Eun HM, McArthur RG, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2:1–4. doi: 10.1016/s0140-6736(88)92941-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Forrest JM, Menser MA, Burgess JA. High frequency of diabetes mellitus in young adults with congenital rubella. Lancet. 1971;2:332–334. doi: 10.1016/s0140-6736(71)90057-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Menser MA, Forrest JM, Bransby RD. Rubella infection and diabetes mellitus. Lancet. 1978;1:57–60. doi: 10.1016/s0140-6736(78)90001-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328:750–754. doi: 10.1136/bmj.328.7442.750. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Kimpimäki T, Kupila A, Hämäläinen AM, Kukko M, Kulmala P, Savola K, Simell T, Keskinen P, Ilonen J, Simell O, et al. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86:4782–4788. doi: 10.1210/jcem.86.10.7907. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Lönnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, Savola K, Muona P, Simell T, Koskela P, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49:1314–1318. doi: 10.2337/diabetes.49.8.1314. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Stene LC, Oikarinen S, Hyöty H, Barriga KJ, Norris JM, Klingensmith G, Hutton JC, Erlich HA, Eisenbarth GS, Rewers M. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–3180. doi: 10.2337/db10-0866. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Hyöty H, Hiltunen M, Knip M, Laakkonen M, Vähäsalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652–657. doi: 10.2337/diab.44.6.652. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:283–292. doi: 10.1172/JCI115573. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol. 2004;110:134–144. doi: 10.1016/j.clim.2003.09.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Simpson M, Brady H, Yin X, Seifert J, Barriga K, Hoffman M, Bugawan T, Barón AE, Sokol RJ, Eisenbarth G, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 2011;54:2779–2788. doi: 10.1007/s00125-011-2278-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–1305. doi: 10.2337/dc10-2456. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–3886. doi: 10.1016/j.vaccine.2005.03.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Lee HS, Burkhardt BR, McLeod W, Smith S, Eberhard C, Lynch K, Hadley D, Rewers M, Simell O, She JX, et al. Biomarker discovery study design for type 1 diabetes in The Environmental Determinants of Diabetes in the Young (TEDDY) study. Diabetes Metab Res Rev. 2014;30:424–434. doi: 10.1002/dmrr.2510. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol. 2010;6:270–277. doi: 10.1038/nrendo.2010.40. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Michels AW, Nakayama M. The anti-insulin trimolecular complex in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:329–334. doi: 10.1097/MED.0b013e32833aba41. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013;1281:16–35. doi: 10.1111/j.1749-6632.2012.06826.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608–617. doi: 10.1002/dmrr.2316. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Pugliese A, Yang M, Kusmarteva I, Heiple T, Vendrame F, Wasserfall C, Rowe P, Moraski JM, Ball S, Jebson L, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15:1–9. doi: 10.1111/pedi.12097. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Michels AW, Eisenbarth GS. Immune intervention in type 1 diabetes. Semin Immunol. 2011;23:214–219. doi: 10.1016/j.smim.2011.07.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Spencer J, Peakman M. Post-mortem analysis of islet pathology in type 1 diabetes illuminates the life and death of the beta cell. Clin Exp Immunol. 2009;155:125–127. doi: 10.1111/j.1365-2249.2008.03864.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Watkins RA, Evans-Molina C, Blum JS, DiMeglio LA. Established and emerging biomarkers for the prediction of type 1 diabetes: a systematic review. Transl Res. 2014;164:110–121. doi: 10.1016/j.trsl.2014.02.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, Eisenbarth GS, Rewers MJ. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Parikka V, Näntö-Salonen K, Saarinen M, Simell T, Ilonen J, Hyöty H, Veijola R, Knip M, Simell O. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. 2012;55:1926–1936. doi: 10.1007/s00125-012-2523-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81:4264–4267. doi: 10.1210/jcem.81.12.8954025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61:179–186. doi: 10.2337/db11-0670. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Miao D, Guyer KM, Dong F, Jiang L, Steck AK, Rewers M, Eisenbarth GS, Yu L. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62:4174–4178. doi: 10.2337/db13-0534. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Sosenko JM, Skyler JS, Krischer JP, Greenbaum CJ, Mahon J, Rafkin LE, Cuthbertson D, Cowie C, Herold K, Eisenbarth G, Palmer JP; Diabetes Prevention Trial-Type 1 Study Group. Glucose excursions between states of glycemia with progression to type 1 diabetes in the diabetes prevention trial-type 1 (DPT-1) Diabetes. 2010;59:2386–2389. doi: 10.2337/db10-0534. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes. 2010;59:679–685. doi: 10.2337/db09-1378. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F, Dentamaro I, Gentile F, Gesualdo M, Maiello M, Modesti PA, et al. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab. 2014;5:364. [ Google Scholar ]
- 64. Ciccone MM, Marchese A, Generali A, Loiodice C, Cortese F, Carbonara R, Scicchitano P, Laviola L, Giorgino F. Interventional therapy in diabetic foot: risk factors, clinical events and prognosis at one year follow-up (a study of 103 cases) Pak J Biol Sci. 2012;15:789–794. doi: 10.3923/pjbs.2012.789.794. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–S80. doi: 10.2337/dc14-S014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Elding Larsson H, Vehik K, Gesualdo P, Akolkar B, Hagopian W, Krischer J, Lernmark Å, Rewers M, Simell O, She JX, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15:118–126. doi: 10.1111/pedi.12066. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Rewers A. Current concepts and controversies in prevention and treatment of diabetic ketoacidosis in children. Curr Diab Rep. 2012;12:524–532. doi: 10.1007/s11892-012-0307-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Dabelea D, Rewers A, Stafford JM, Standiford DA, Lawrence JM, Saydah S, Imperatore G, D’Agostino RB, Mayer-Davis EJ, Pihoker C; SEARCH for Diabetes in Youth Study Group. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133:e938–e945. doi: 10.1542/peds.2013-2795. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT) The DCCT Research Group. J Clin Endocrinol Metab. 1987;65:30–36. doi: 10.1210/jcem-65-1-30. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Sjöberg S, Gjötterberg M, Berglund L, Möller E, Ostman J. Residual C-peptide excretion is associated with a better long-term glycemic control and slower progress of retinopathy in type I (insulin-dependent) diabetes mellitus. J Diabet Complications. 1991;5:18–22. doi: 10.1016/0891-6632(91)90005-a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Nakanishi K, Kobayashi T, Miyashita H, Ohkubo M, Sugimoto T, Murase T, Kosaka K, Inouye K, Kono M. Relationships among islet cell antibodies, residual beta-cell function, and metabolic control in patients with insulin-dependent diabetes mellitus of long duration: use of a sensitive C-peptide radioimmunoassay. Metabolism. 1990;39:925–930. doi: 10.1016/0026-0495(90)90302-s. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Nakanishi K, Watanabe C. Rate of beta-cell destruction in type 1 diabetes influences the development of diabetic retinopathy: protective effect of residual beta-cell function for more than 10 years. J Clin Endocrinol Metab. 2008;93:4759–4766. doi: 10.1210/jc.2008-1209. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Panero F, Novelli G, Zucco C, Fornengo P, Perotto M, Segre O, Grassi G, Cavallo-Perin P, Bruno G. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care. 2009;32:301–305. doi: 10.2337/dc08-1241. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Mühlhauser I, Overmann H, Bender R, Bott U, Berger M. Risk factors of severe hypoglycaemia in adult patients with Type I diabetes--a prospective population based study. Diabetologia. 1998;41:1274–1282. doi: 10.1007/s001250051065. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Mortensen HB, Swift PG, Holl RW, Hougaard P, Hansen L, Bjoerndalen H, de Beaufort CE, Knip M. Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes. 2010;11:218–226. doi: 10.1111/j.1399-5448.2009.00566.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Picardi A, Visalli N, Lauria A, Suraci C, Buzzetti R, Merola MK, Manfrini S, Guglielmi C, Gentilucci UV, Pitocco D, et al. Metabolic factors affecting residual beta cell function assessed by C-peptide secretion in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2006;38:668–672. doi: 10.1055/s-2006-954586. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Böber E, Dündar B, Büyükgebiz A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2001;14:435–441. doi: 10.1515/jpem.2001.14.4.435. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Bonfanti R, Bognetti E, Meschi F, Brunelli A, Riva MC, Pastore MR, Calori G, Chiumello G. Residual beta-cell function and spontaneous clinical remission in type 1 diabetes mellitus: the role of puberty. Acta Diabetol. 1998;35:91–95. doi: 10.1007/s005920050110. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Komulainen J, Knip M, Lounamaa R, Vähäsalo P, Karjalainen J, Sabbah E, Akerblom HK. Poor beta-cell function after the clinical manifestation of type 1 diabetes in children initially positive for islet cell specific autoantibodies. The Childhood Diabetes in Finland Study Group. Diabet Med. 1997;14:532–537. doi: 10.1002/(SICI)1096-9136(199707)14:7<532::AID-DIA403>3.0.CO;2-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Egger M, Gschwend S, Smith GD, Zuppinger K. Increasing incidence of hypoglycemic coma in children with IDDM. Diabetes Care. 1991;14:1001–1005. doi: 10.2337/diacare.14.11.1001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Strong K, Wald N, Miller A, Alwan A. Current concepts in screening for noncommunicable disease: World Health Organization Consultation Group Report on methodology of noncommunicable disease screening. J Med Screen. 2005;12:12–19. doi: 10.1258/0969141053279086. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Clague A, Thomas A. Neonatal biochemical screening for disease. Clin Chim Acta. 2002;315:99–110. doi: 10.1016/s0009-8981(01)00716-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Skyler JS. Primary and secondary prevention of Type 1 diabetes. Diabet Med. 2013;30:161–169. doi: 10.1111/dme.12100. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Michels AW, von Herrath M. 2011 Update: antigen-specific therapy in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2011;18:235–240. doi: 10.1097/MED.0b013e32834803ae. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–419. doi: 10.1038/nri1370. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Weiner HL, Mayer LF. Oral tolerance: mechanisms and applications. Introduction. Ann N Y Acad Sci. 1996;778:xiii–xviii. doi: 10.1111/j.1749-6632.1996.tb21109.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Vehik K, Cuthbertson D, Ruhlig H, Schatz DA, Peakman M, Krischer JP; DPT-1 and TrialNet Study Groups. Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care. 2011;34:1585–1590. doi: 10.2337/dc11-0523. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Vandemeulebroucke E, Gorus FK, Decochez K, Weets I, Keymeulen B, De Block C, Tits J, Pipeleers DG, Mathieu C; Belgian Diabetes Registry. Insulin treatment in IA-2A-positive relatives of type 1 diabetic patients. Diabetes Metab. 2009;35:319–327. doi: 10.1016/j.diabet.2009.02.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest. 2003;111:1365–1371. doi: 10.1172/JCI17166. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 96. Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, Couper JJ, Colman PG. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27:2348–2355. doi: 10.2337/diacare.27.10.2348. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Näntö-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipilä JI, Haavisto L, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Böhmer KP, Kolb H, Kuglin B, Zielasek J, Hübinger A, Lampeter EF, Weber B, Kolb-Bachofen V, Jastram HU, Bertrams J. Linear loss of insulin secretory capacity during the last six months preceding IDDM. No effect of antiedematous therapy with ketotifen. Diabetes Care. 1994;17:138–141. doi: 10.2337/diacare.17.2.138. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Carel JC, Boltard C, Eisenbarth G, Bach JF, Bougneres PF. Cyclosporine Delays but does not Prevent Clinical Onset in Glucose Intolerant Pre-type 1 Diabetic Children. J Autoimmun. 1996;9:739–745. doi: 10.1006/jaut.1996.0096. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal Bacille Calmette-Guerin vaccination and type 1 diabetes. Diabetes Care. 2005;28:1204–1206. doi: 10.2337/diacare.28.5.1204. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Dulin WE, Wyse BM. Reversal of streptozotocin diabetes with nicotinamide. Proc Soc Exp Biol Med. 1969;130:992–994. doi: 10.3181/00379727-130-33707. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Yamada K, Nonaka K, Hanafusa T, Miyazaki A, Toyoshima H, Tarui S. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. An observation in nonobese diabetic (NOD) mice. Diabetes. 1982;31:749–753. doi: 10.2337/diab.31.9.749. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Lazarow A. Protection against alloxan diabetes. Anat Rec. 1947;97:353. [ PubMed ] [ Google Scholar ]
- 105. Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [ DOI ] [ PubMed ] [ Google Scholar ]
- 106. Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. doi: 10.2337/db13-0345. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, Bode B, Aronoff S, Holland C, Carlin D, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–497. doi: 10.1016/S0140-6736(11)60931-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008;359:1909–1920. doi: 10.1056/NEJMoa0804328. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–442. doi: 10.1056/NEJMoa1107096. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Michels AW. Targeting the trimolecular complex. Clin Immunol. 2013;149:339–344. doi: 10.1016/j.clim.2013.02.020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Michels AW, Ostrov DA, Zhang L, Nakayama M, Fuse M, McDaniel K, Roep BO, Gottlieb PA, Atkinson MA, Eisenbarth GS. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol. 2011;187:5921–5930. doi: 10.4049/jimmunol.1100746. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Zhang L, Crawford F, Yu L, Michels A, Nakayama M, Davidson HW, Kappler JW, Eisenbarth GS. Monoclonal antibody blocking the recognition of an insulin peptide-MHC complex modulates type 1 diabetes. Proc Natl Acad Sci USA. 2014;111:2656–2661. doi: 10.1073/pnas.1323436111. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 118. Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottlieb PA, Putnam AL, Gaur A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–180. doi: 10.1172/JCI8525. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 119. Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 120. Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501–1510. doi: 10.1084/jem.20110574. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (961.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 March 2017
Type 1 diabetes mellitus
- Anastasia Katsarou 1 ,
- Soffia Gudbjörnsdottir 2 , 3 ,
- Araz Rawshani 2 , 3 ,
- Dana Dabelea 4 ,
- Ezio Bonifacio 5 ,
- Barbara J. Anderson 6 ,
- Laura M. Jacobsen 7 ,
- Desmond A. Schatz 7 &
- Åke Lernmark 1
Nature Reviews Disease Primers volume 3 , Article number: 17016 ( 2017 ) Cite this article
61k Accesses
812 Citations
84 Altmetric
Metrics details
- Autoimmune diseases
- Diabetic nephropathy
- Diagnostic markers
- Insulin signalling
- Type 1 diabetes
Type 1 diabetes mellitus (T1DM), also known as autoimmune diabetes, is a chronic disease characterized by insulin deficiency due to pancreatic β-cell loss and leads to hyperglycaemia. Although the age of symptomatic onset is usually during childhood or adolescence, symptoms can sometimes develop much later. Although the aetiology of T1DM is not completely understood, the pathogenesis of the disease is thought to involve T cell-mediated destruction of β-cells. Islet-targeting autoantibodies that target insulin, 65 kDa glutamic acid decarboxylase, insulinoma-associated protein 2 and zinc transporter 8 — all of which are proteins associated with secretory granules in β-cells — are biomarkers of T1DM-associated autoimmunity that are found months to years before symptom onset, and can be used to identify and study individuals who are at risk of developing T1DM. The type of autoantibody that appears first depends on the environmental trigger and on genetic factors. The pathogenesis of T1DM can be divided into three stages depending on the absence or presence of hyperglycaemia and hyperglycaemia-associated symptoms (such as polyuria and thirst). A cure is not available, and patients depend on lifelong insulin injections; novel approaches to insulin treatment, such as insulin pumps, continuous glucose monitoring and hybrid closed-loop systems, are in development. Although intensive glycaemic control has reduced the incidence of microvascular and macrovascular complications, the majority of patients with T1DM are still developing these complications. Major research efforts are needed to achieve early diagnosis, prevent β-cell loss and develop better treatment options to improve the quality of life and prognosis of those affected.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
A perspective on treating type 1 diabetes mellitus before insulin is needed

Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure
Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy
SEARCH Study Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control. Clin. Trials 25 , 458–471 (2004).
Google Scholar
Gepts, W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14 , 619–633 (1965). This paper represents the hallmark investigation and rediscovery of insulitis in individuals who died shortly after the clinical diagnosis of T1DM.
Eisenbarth, G. S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 314 , 1360–1368 (1986). This review describes the concept of T1DM pathogenesis that eventually resulted in the staging of T1DM depicted in Figure 1 of this Primer.
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383 , 69–82 (2014).
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 38 , S8–S16 (2015).
Ziegler, A. G., Hummel, M., Schenker, M. & Bonifacio, E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 48 , 460–468 (1999).
Ilonen, J. et al . Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 62 , 3636–3640 (2013). This is the first investigation to dissect the temporal pattern of the first-appearing β-cell-targeting autoantibody as a biomarker of T1DM.
Krischer, J. P. et al . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 58 , 980–987 (2015).
Ziegler, A. G. et al . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309 , 2473–2479 (2013). These authors merge data from three independent longitudinal studies that followed children from birth and demonstrate that the presence of multiple β-cell-targeting autoantibodies inevitably leads to the clinical onset of T1DM.
Rewers, M. et al . Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 39 , 807–812 (1996).
Nejentsev, S. et al . Population-based genetic screening for the estimation of type 1 diabetes mellitus risk in Finland: selective genotyping of markers in the HLA-DQB1, HLA-DQA1 and HLA-DRB1 loci. Diabet. Med. 16 , 985–992 (1999).
TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr. Diabetes 8 , 286–298 (2007).
Insel, R. A. et al . Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38 , 1964–1974 (2015).
International Diabetes Federation. IDF diabetes atlas. IDF http://www.diabetesatlas.org/component/attachments/?task=download&id=116 (2015).
Diaz-Valencia, P. A., Bougneres, P. & Valleron, A. J. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health 15 , 255 (2015).
Askar, M. et al . 16th IHIW: global distribution of extended HLA haplotypes. Int. J. Immunogenet. 40 , 31–38 (2013).
Erlich, H. A. et al . HLA class II alleles and susceptibility and resistance to insulin dependent diabetes mellitus in Mexican-American families. Nat. Genet. 3 , 358–364 (1993).
Delli, A. J. et al . Type 1 diabetes patients born to immigrants to Sweden increase their native diabetes risk and differ from Swedish patients in HLA types and islet autoantibodies. Pediatr. Diabetes 11 , 513–520 (2010).
Serrano-Rios, M., Goday, A. & Martinez Larrad, T. Migrant populations and the incidence of type 1 diabetes mellitus: an overview of the literature with a focus on the Spanish-heritage countries in Latin America. Diabetes Metab. Res. Rev. 15 , 113–132 (1999).
Kondrashova, A., Seiskari, T., Ilonen, J., Knip, M. & Hyoty, H. The ‘hygiene hypothesis’ and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. APMIS 121 , 478–493 (2013). Along with other studies by the same authors, this study demonstrates the importance of gene–environment interactions in the risk of developing both allergies and cell-specific autoimmune diseases such as T1DM.
Patterson, C. C., Dahlquist, G. G., Gyurus, E., Green, A. & Soltesz, G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 373 , 2027–2033 (2009).
Thunander, M. et al . Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res. Clin. Pract. 82 , 247–255 (2008).
Turner, R. et al . UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 350 , 1288–1293 (1997).
Landin-Olsson, M., Nilsson, K. O., Lernmark, Å. & Sundkvist, G. Islet cell antibodies and fasting C-peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologia 33 , 561–568 (1990).
Hagopian, W. A. et al . Quantitative assay using recombinant human islet glutamic acid decarboxylase (GAD65) shows that 64K autoantibody positivity at onset predicts diabetes type. J. Clin. Invest. 91 , 368–374 (1993).
Tuomi, T. et al . Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes 42 , 359–362 (1993).
Svensson, J., Carstensen, B., Mortensen, H. B. & Borch-Johnsen, K. Early childhood risk factors associated with type 1 diabetes — is gender important? Eur. J. Epidemiol. 20 , 429–434 (2005).
Patterson, C. C. et al . Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia 55 , 2142–2147 (2012).
Soltesz, G., Patterson, C. C. & Dahlquist, G. Worldwide childhood type 1 diabetes incidence — what can we learn from epidemiology? Pediatr. Diabetes 8 (Suppl. 6), 6–14 (2007).
Rawshani, A. et al . The incidence of diabetes among 0–34 year olds in Sweden: new data and better methods. Diabetologia 57 , 1375–1381 (2014).
Bonifacio, E. et al . Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J. Clin. Endocrinol. Metab. 95 , 3360–3367 (2010).
Vehik, K. et al . Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 34 , 1897–1901 (2011).
Sosenko, J. M. et al . The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 36 , 2615–2620 (2013).
Xu, P. & Krischer, J. P. Prognostic classification factors associated with development of multiple autoantibodies, dysglycemia, and type 1 diabetes — a recursive partitioning analysis. Diabetes Care 39 , 1036–1044 (2016).
Bonifacio, E. Predicting type 1 diabetes using biomarkers. Diabetes Care 38 , 989–996 (2015).
LaGasse, J. M. et al . Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care 25 , 505–511 (2002).
Schlosser, M. et al . The Karlsburg type 1 diabetes risk study of a normal schoolchild population: association of β-cell autoantibodies and human leukocyte antigen-DQB1 alleles in antibody-positive individuals. J. Clin. Endocrinol. Metab. 87 , 2254–2261 (2002).
Mahon, J. L. et al . The TrialNet Natural History Study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr. Diabetes 10 , 97–104 (2009).
Gorus, F. K. et al . Influence of age on the associations among insulin autoantibodies, islet cell antibodies, and HLA DAQ1*0301-DQB1*0302 in siblings of patients with type 1 (insulin-dependent) diabetes mellitus. Belgian Diabetes Registry. J. Clin. Endocrinol. Metab. 78 , 1172–1178 (1994).
Rolandsson, O. et al . Glutamate decarboxylase (GAD65) and tyrosine phosphatase-like protein (IA-2) autoantibodies index in a regional population is related to glucose intolerance and body mass index. Diabetologia 42 , 555–559 (1999).
Rewers, M. et al . β-cell autoantibodies in infants and toddlers without IDDM relatives: Diabetes Autoimmunity Study in the Young (DAISY). J. Autoimmun. 9 , 405–410 (1996).
Hagopian, W. A. et al . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr. Diabetes 12 , 733–743 (2011).
Wenzlau, J. M. et al . A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57 , 2693–2697 (2008).
Savola, K. et al . IA-2 antibodies — a sensitive marker of IDDM with clinical onset in childhood and adolescence. Childhood Diabetes in Finland Study Group. Diabetologia 41 , 424–429 (1998).
Lampasona, V. et al . Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: non insulin requiring autoimmune diabetes (NIRAD) 4. Diabetes Care 33 , 104–108 (2010).
Skarstrand, H. et al . Zinc transporter 8 (ZnT8) autoantibody epitope specificity and affinity examined with recombinant ZnT8 variant proteins in specific ZnT8R and ZnT8W autoantibody-positive type 1 diabetes patients. Clin. Exp. Immunol. 179 , 220–229 (2015).
Tuomilehto, J. The emerging global epidemic of type 1 diabetes. Curr. Diab. Rep. 13 , 795–804 (2013).
Redondo, M. J., Jeffrey, J., Fain, P. R., Eisenbarth, G. S. & Orban, T. Concordance for islet autoimmunity among monozygotic twins. N. Engl. J. Med. 359 , 2849–2850 (2008).
Nerup, J. et al . HL-A antigens and diabetes mellitus. Lancet 2 , 864–866 (1974).
Singal, D. P. & Blajchman, M. A. Histocompatibility (HL-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes 22 , 429–432 (1973).
Cudworth, A. G. & Woodrow, J. C. Evidence for HL-A-linked genes in “juvenile” diabetes mellitus. Br. Med. J. 3 , 133–135 (1975).
Erlich, H. A. et al . Next generation sequencing reveals the association of DRB3*02:02 with type 1 diabetes. Diabetes 62 , 2618–2622 (2013).
Caillat-Zucman, S. et al . Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J. Clin. Invest. 90 , 2242–2250 (1992).
Cucca, F. et al . The distribution of DR4 haplotypes in Sardinia suggests a primary association of type I diabetes with DRB1 and DQB1 loci. Hum. Immunol. 43 , 301–308 (1995).
Zhao, L. P. et al . Next-generation sequencing reveals that HLA-DRB3, -DRB4, and -DRB5 may be associated with islet autoantibodies and risk for childhood type 1 diabetes. Diabetes 65 , 710–718 (2016).
Graham, J. et al . Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 51 , 1346–1355 (2002). This major investigation of patients with newly diagnosed T1DM (1–34 years of age) demonstrates that the age-dependent onset of T1DM is strongly related to the presence of β-cell-targeting autoantibodies, which is associated with specific HLA-DR-DQ genotypes.
Dahlquist, G. et al . The epidemiology of diabetes in Swedish children 0–14 years — a six-year prospective study. Diabetologia 28 , 802–808 (1985).
Parkkola, A., Harkonen, T., Ryhanen, S. J., Ilonen, J. & Knip, M. Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care 36 , 348–354 (2013).
Torn, C. et al . Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 64 , 1818–1829 (2015).
Wester, A. et al . An increased diagnostic sensitivity of truncated GAD65 autoantibodies in type 1 diabetes may be related to HLA-DQ8. Diabetes 66 , 735–740 (2016).
Cooper, J. D. et al . Confirmation of novel type 1 diabetes risk loci in families. Diabetologia 55 , 996–1000 (2012).
Pociot, F. & Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 387 , 2331–2339 (2016).
Rich, S. S. et al . Overview of the Type I Diabetes Genetics Consortium. Genes Immun. 10 , S1–S4 (2009).
Bell, G. I., Pictet, R. & Rutter, W. J. Analysis of the regions flanking the human insulin gene and sequence of an Alu family member. Nucleic Acids Res. 8 , 4091–4109 (1980).
Polychronakos, C. & Li, Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat. Rev. Genet. 12 , 781–792 (2011).
Pugliese, A. et al . The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15 , 293–297 (1997).
Beyerlein, A., Donnachie, E., Jergens, S. & Ziegler, A. G. Infections in early life and development of type 1 diabetes. JAMA 315 , 1899–1901 (2016).
Ashton, M. P. et al . Incomplete immune response to coxsackie B viruses associates with early autoimmunity against insulin. Sci. Rep. 6 , 32899 (2016).
Hyoty, H. Viruses in type 1 diabetes. Pediatr. Diabetes 17 (Suppl. 22), 56–64 (2016).
Knip, M., Virtanen, S. M. & Akerblom, H. K. Infant feeding and the risk of type 1 diabetes. Am. J. Clin. Nutr. 91 , 1506S–1513S (2010).
La Torre, D. et al . Decreased cord-blood phospholipids in young age-at-onset type 1 diabetes. Diabetes 62 , 3951–3956 (2013).
Oresic, M. et al . Cord serum lipidome in prediction of islet autoimmunity and type 1 diabetes. Diabetes 62 , 3268–3274 (2013).
Lynch, K. F. et al . Cord blood islet autoantibodies and seasonal association with the type 1 diabetes high-risk genotype. J. Perinatol. 28 , 211–217 (2008).
Resic Lindehammer, S. et al . Seroconversion to islet autoantibodies after enterovirus infection in early pregnancy. Viral Immunol. 25 , 254–261 (2012).
Viskari, H. R. et al . Maternal first-trimester enterovirus infection and future risk of type 1 diabetes in the exposed fetus. Diabetes 51 , 2568–2571 (2002).
Bonifacio, E. et al . Maternal type 1 diabetes reduces the risk of islet autoantibodies: relationships with birthweight and maternal HbA1c . Diabetologia 51 , 1245–1252 (2008).
Hofer, J. et al . Elevated proportions of recent thymic emigrants in children and adolescents with type 1 diabetes. Rejuvenation Res. 12 , 311–320 (2009).
Wong, F. S. How does B-cell tolerance contribute to the protective effects of diabetes following induced mixed chimerism in autoimmune diabetes? Diabetes 63 , 1855–1857 (2014).
Roep, B. O. & Peakman, M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb. Perspect. Med. 2 , a007781 (2012).
Oling, V., Reijonen, H., Simell, O., Knip, M. & Ilonen, J. Autoantigen-specific memory CD4 + T cells are prevalent early in progression to type 1 diabetes. Cell. Immunol. 273 , 133–139 (2012).
van Lummel, M. et al . Post-translational modification of HLA-DQ binding islet-autoantigens in type 1 diabetes. Diabetes 63 , 237–247 (2014).
Delong, T. et al . Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351 , 711–714 (2016).
McLaughlin, R. J., Spindler, M. P., van Lummel, M. & Roep, B. O. Where, how, and when: positioning posttranslational modification within type 1 diabetes pathogenesis. Curr. Diab. Rep. 16 , 63 (2016).
Yang, J. et al . Autoreactive T cells specific for insulin B:11–23 recognize a low-affinity peptide register in human subjects with autoimmune diabetes. Proc. Natl Acad. Sci. USA 111 , 14840–14845 (2014).
Yang, J., James, E. A., Sanda, S., Greenbaum, C. & Kwok, W. W. CD4 + T cells recognize diverse epitopes within GAD65: implications for repertoire development and diabetes monitoring. Immunology 138 , 269–279 (2013).
Miani, M., Colli, M. L., Ladriere, L., Cnop, M. & Eizirik, D. L. Mild endoplasmic reticulum stress augments the proinflammatory effect of IL-1β in pancreatic rat β-cells via the IRE1α/XBP1s pathway. Endocrinology 153 , 3017–3028 (2012).
Eizirik, D. L., Miani, M. & Cardozo, A. K. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 56 , 234–241 (2013).
James, E. A. et al . Immunology of Diabetes Society T-cell workshop: HLA class II tetramer-directed epitope validation initiative. Diabetes Metab. Res. Rev. 27 , 727–736 (2011).
McGinty, J. W. et al . Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes 63 , 3033–3040 (2014).
Wiberg, A. et al . Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin. Exp. Immunol. 182 , 278–288 (2015).
Babon, J. A. et al . Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med. 22 , 1482–1487 (2016).
Campbell-Thompson, M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr. Diabetes 16 , 320–330 (2015).
In't Veld, P. et al . Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56 , 2400–2404 (2007).
Richardson, S. J. et al . Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 59 , 2448–2458 (2016).
Sosenko, J. M. et al . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 38 , 271–276 (2015).
Helminen, O. et al . OGTT and random plasma glucose in the prediction of type 1 diabetes and time to diagnosis. Diabetologia 58 , 1787–1796 (2015).
Sosenko, J. M. et al . Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes 62 , 4179–4183 (2013).
Helminen, O. et al . HbA1c predicts time to diagnosis of type 1 diabetes in children at risk. Diabetes 64 , 1719–1727 (2015).
Magnuson, A. M. et al . Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc. Natl Acad. Sci. USA 112 , 1511–1516 (2015).
Engin, F. et al . Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci. Transl Med. 5 , 211ra156 (2013).
Mordes, J. P., Bortell, R., Blankenhorn, E. P., Rossini, A. A. & Greiner, D. L. Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J. 45 , 278–291 (2004).
Kaldunski, M. et al . Identification of a serum-induced transcriptional signature associated with type 1 diabetes in the BioBreeding rat. Diabetes 59 , 2375–2385 (2010).
Bogdani, M. et al . BioBreeding rat islets exhibit reduced antioxidative defense and N-acetyl cysteine treatment delays type 1 diabetes. J. Endocrinol. 216 , 111–123 (2013).
Krogvold, L. et al . Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia 59 , 492–501 (2016).
Imagawa, A. et al . Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes 50 , 1269–1273 (2001).
Krogvold, L. et al . Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 57 , 841–843 (2014).
Bottazzo, G. F. et al . In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N. Engl. J. Med. 313 , 353–360 (1985).
Lernmark, Å. et al . Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch. 425 , 631–640 (1995).
Bottazzo, G. F. & Lendrum, R. Separate autoantibodies to human pancreatic glucagon and somatostatin cells. Lancet 2 , 873–876 (1976).
Pugliese, A. et al . The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr. Diabetes 15 , 1–9 (2014).
Akirav, E., Kushner, J. A. & Herold, K. C. β-Cell mass and type 1 diabetes: going, going, gone? Diabetes 57 , 2883–2888 (2008).
Bjork, E. et al . Glucose regulation of the autoantigen GAD65 in human pancreatic islets. J. Clin. Endocrinol. Metab. 75 , 1574–1576 (1992).
Melendez-Ramirez, L. Y., Richards, R. J. & Cefalu, W. T. Complications of type 1 diabetes. Endocrinol. Metab. Clin. North Am. 39 , 625–640 (2010).
Miki, T., Yuda, S., Kouzu, H. & Miura, T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail. Rev. 18 , 149–166 (2013).
Lind, M. et al . Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 378 , 140–146 (2011).
Rosengren, A. et al . Long-term excess risk of heart failure in people with type 1 diabetes: a prospective case-control study. Lancet Diabetes Endocrinol. 3 , 876–885 (2015).
McMurray, J. J., Gerstein, H. C., Holman, R. R. & Pfeffer, M. A. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2 , 843–851 (2014).
Jacobson, A. M. et al . Long-term effect of diabetes and its treatment on cognitive function. N. Engl. J. Med. 356 , 1842–1852 (2007). This comprehensive investigation demonstrates that long-term T1DM and recurrent hypoglycaemic episodes do not affect cognitive function.
Shah, A. D. et al . Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 3 , 105–113 (2015).
Zinman, B. et al . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373 , 2117–2128 (2015).
Wanner, C. et al . Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375 , 323–334 (2016).
Sattar, N., McLaren, J., Kristensen, S. L., Preiss, D. & McMurray, J. J. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia 59 , 1333–1339 (2016).
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker versus diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288 , 2981–2997 (2002).
de Ferranti, S. D. et al . Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 37 , 2843–2863 (2014).
de Ferranti, S. D. et al . Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 130 , 1110–1130 (2014).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107 , 1058–1070 (2010).
Paneni, F., Beckman, J. A., Creager, M. A. & Cosentino, F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur. Heart J. 34 , 2436–2443 (2013).
Saydah, S. H. et al . Trends and characteristics of self-reported case presentation of diabetes diagnosis among youth from 2002 to 2010: findings from the SEARCH for diabetes in youth study. Diabetes Care 38 , e84–e85 (2015).
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 39 , S13–S22 (2016).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 36 , S67–S74 (2013).
Hattersley, A. T. & Ashcroft, F. M. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 54 , 2503–2513 (2005).
Flanagan, S. E. et al . Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat. Genet. 46 , 812–814 (2014).
Johnson, M. B., Hattersley, A. T. & Flanagan, S. E. Monogenic autoimmune diseases of the endocrine system. Lancet Diabetes Endocrinol. 4 , 862–872 (2016).
Johansson, B. B. et al . Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia 60 , 625 (2017).
Mire-Sluis, A. R., Gaines Das, R. & Lernmark, Å. The World Health Organization International Collaborative Study for islet cell antibodies. Diabetologia 43 , 1282–1292 (2000).
Strauss, R. S. & Pollack, H. A. Epidemic increase in childhood overweight, 1986–1998. JAMA 286 , 2845–2848 (2001).
Liu, L. L. et al . Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr. Diabetes 11 , 4–11 (2010).
Carlsson, A. et al . Low risk HLA-DQ and increased body mass index in newly diagnosed type 1 diabetes children in the Better Diabetes Diagnosis study in Sweden. Int. J. Obes. (Lond.) 36 , 718–724 (2012).
Dahlquist, G. et al . The Swedish childhood diabetes study — results from a nine year case register and a one year case-referent study indicating that type 1 (insulin-dependent) diabetes mellitus is associated with both type 2 (non-insulin-dependent) diabetes mellitus and autoimmune disorders. Diabetologia 32 , 2–6 (1989).
Pinhas-Hamiel, O., Dolan, L. M. & Zeitler, P. S. Diabetic ketoacidosis among obese African-American adolescents with NIDDM. Diabetes Care 20 , 484–486 (1997).
Sellers, E. A. & Dean, H. J. Diabetic ketoacidosis: a complication of type 2 diabetes in Canadian aboriginal youth. Diabetes Care 23 , 1202–1204 (2000).
Hathout, E. H., Thomas, W., El-Shahawy, M., Nahab, F. & Mace, J. W. Diabetic autoimmune markers in children and adolescents with type 2 diabetes. Pediatrics 107 , E102 (2001).
Libman, I. M. & Becker, D. J. Coexistence of type 1 and type 2 diabetes mellitus: “double” diabetes? Pediatr. Diabetes 4 , 110–113 (2003).
Dabelea, D. et al . Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care 34 , 1628–1633 (2011).
Dabelea, D. et al . Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 54 , 78–86 (2011).
Nathan, D. M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37 , 9–16 (2014).
Lind, M. et al . Glycemic control and excess mortality in type 1 diabetes. N. Engl. J. Med. 371 , 1972–1982 (2014). This registry-based observational study finds that the risk of death from any cause or from cardiovascular causes among patients with T1DM who have a HbA1c level of ≤6.9% is twice as high as the risk among matched controls.
Livingstone, S. J. et al . Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA 313 , 37–44 (2015).
Huxley, R. R., Peters, S. A., Mishra, G. D. & Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 3 , 198–206 (2015).
Lachin, J. M., Genuth, S., Nathan, D. M., Zinman, B. & Rutledge, B. N. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial — revisited. Diabetes 57 , 995–1001 (2008).
Anderzen, J., Samuelsson, U., Gudbjornsdottir, S., Hanberger, L. & Akesson, K. Teenagers with poor metabolic control already have a higher risk of microvascular complications as young adults. J. Diabetes Complicat. 30 , 533–536 (2016).
Yau, J. W. et al . Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35 , 556–564 (2012).
Frank, R. N. Diabetic retinopathy. N. Engl. J. Med. 350 , 48–58 (2004).
Raile, K. et al . Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 30 , 2523–2528 (2007).
Gross, J. L. et al . Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28 , 164–176 (2005).
Valmadrid, C. T., Klein, R., Moss, S. E. & Klein, B. E. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch. Intern. Med. 160 , 1093–1100 (2000).
Vasan, R. S. et al . Impact of high-normal blood pressure on the risk of cardiovascular disease. N. Engl. J. Med. 345 , 1291–1297 (2001).
Hovind, P. et al . Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 328 , 1105 (2004).
Caramori, M. L., Fioretto, P. & Mauer, M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 49 , 1399–1408 (2000).
Perkins, B. A. et al . Regression of microalbuminuria in type 1 diabetes. N. Engl. J. Med. 348 , 2285–2293 (2003).
Voulgari, C. et al . The association between cardiac autonomic neuropathy with metabolic and other factors in subjects with type 1 and type 2 diabetes. J. Diabetes Complicat. 25 , 159–167 (2011).
Gerstein, H. C. Diabetes: dysglycaemia as a cause of cardiovascular outcomes. Nat. Rev. Endocrinol. 11 , 508–510 (2015).
Gerstein, H. C. & Werstuck, G. H. Dysglycaemia, vasculopenia, and the chronic consequences of diabetes. Lancet Diabetes Endocrinol. 1 , 71–78 (2013).
Nathan, D. M. et al . Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med. 348 , 2294–2303 (2003).
Dabelea, D. et al . Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes 52 , 2833–2839 (2003).
Jarvisalo, M. J. et al . Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes 51 , 493–498 (2002).
Margeirsdottir, H. D., Stensaeth, K. H., Larsen, J. R., Brunborg, C. & Dahl-Jorgensen, K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care 33 , 2043–2048 (2010).
Secrest, A. M., Becker, D. J., Kelsey, S. F., Laporte, R. E. & Orchard, T. J. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 59 , 3216–3222 (2010).
Soedamah-Muthu, S. S. et al . High risk of cardiovascular disease in patients with type diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 29 , 798–804 (2006).
Jonasson, J. M. et al . Risks of nontraumatic lower-extremity amputations in patients with type 1 diabetes: a population-based cohort study in Sweden. Diabetes Care 31 , 1536–1540 (2008).
Deckert, T., Poulsen, J. E. & Larsen, M. The prognosis of insulin dependent diabetes mellitus and the importance of supervision. Acta Med. Scand. Suppl. 624 , 48–53 (1979).
Keenan, H. A. et al . Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist study. Diabetes 59 , 2846–2853 (2010).
Hahl, J., Simell, T., Ilonen, J., Knip, M. & Simell, O. Costs of predicting IDDM. Diabetologia 41 , 79–85 (1998).
Kukko, M. et al . Geographical variation in risk HLA-DQB1 genotypes for type 1 diabetes and signs of β-cell autoimmunity in a high-incidence country. Diabetes Care 27 , 676–681 (2004).
Larsson, H. E. et al . Diabetes-associated HLA genotypes affect birthweight in the general population. Diabetologia 48 , 1484–1491 (2005).
Carmichael, S. K. et al . Prospective assessment in newborns of diabetes autoimmunity (PANDA): maternal understanding of infant diabetes risk. Genet. Med. 5 , 77–83 (2003).
Elding Larsson, H. et al . Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 34 , 2347–2352 (2011).
Elding Larsson, H. et al . Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr. Diabetes 15 , 118–126 (2014).
Raab, J. et al . Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 6 , e011144 (2016).
Knip, M. et al . Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA 311 , 2279–2287 (2014).
Bonifacio, E. et al . Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA 313 , 1541–1549 (2015).
Chase, H. P. et al . Nutritional Intervention to Prevent (NIP) type 1 diabetes a pilot trial. Infant Child Adolesc. Nutr. 1 , 98–107 (2009).
Skyler, J. S. et al . Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial — Type 1. Diabetes Care 28 , 1068–1076 (2005).
Vehik, K. et al . Long-term outcome of individuals treated with oral insulin: Diabetes Prevention Trial-Type 1 (DPT-1) oral insulin trial. Diabetes Care 34 , 1585–1590 (2011).
American Diabetes Association. 5. Glycemic targets. Diabetes Care 39 , S39–S46 (2016).
Rewers, A. et al . Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics 121 , e1258–e1266 (2008).
Wolfsdorf, J., Glaser, N. & Sperling, M. A. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care 29 , 1150–1159 (2006).
Tonyushkina, K. N., Visintainer, P. F., Jasinski, C. F., Wadzinski, T. L. & Allen, H. F. Site of initial diabetes education does not affect metabolic outcomes in children with T1DM. Pediatr. Diabetes 15 , 135–141 (2014).
Jasinski, C. F., Rodriguez-Monguio, R., Tonyushkina, K. & Allen, H. Healthcare cost of type 1 diabetes mellitus in new-onset children in a hospital compared to an outpatient setting. BMC Pediatr. 13 , 55 (2013).
Lowes, L. & Gregory, J. W. Management of newly diagnosed diabetes: home or hospital? Arch. Dis. Child. 89 , 934–937 (2004).
Simell, T., Kaprio, E. A., Maenpaa, J., Tuominen, J. & Simell, O. Randomised prospective study of short-term and long-term initial stay in hospital by children with diabetes mellitus. Lancet 337 , 656–660 (1991).
Dhaliwal, R. & Weinstock, R. S. Management of type 1 diabetes in older adults. Diabetes Spectr. 27 , 9–20 (2014).
Auer, R. N. Hypoglycemic brain damage. Metab. Brain Dis. 19 , 169–175 (2004).
Auer, R. N. Hypoglycemic brain damage. Forensic Sci. Int. 146 , 105–110 (2004).
Barnard, K., Thomas, S., Royle, P., Noyes, K. & Waugh, N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr. 10 , 50 (2010).
Miller, K. M. et al . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 36 , 2009–2014 (2013).
Skyler, J. S. Immune intervention for type 1 diabetes mellitus. Int. J. Clin. Pract. Suppl. 65 , 61–70 (2011).
The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 37 , 1574–1582 (1988).
Cook, J. J. et al . Double-blind controlled trial of azathioprine in children with newly diagnosed type I diabetes. Diabetes 38 , 779–783 (1989).
Herold, K. C. et al . Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346 , 1692–1698 (2002).
Keymeulen, B. et al . Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352 , 2598–2608 (2005).
Pescovitz, M. D. et al . Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N. Engl. J. Med. 361 , 2143–2152 (2009). This paper describes the first randomized controlled trial of a monoclonal antibody against the B cell surface protein CD20; this antibody preserved residual β-cell function better than did T cell-targeting antibodies, which contradicts the long-held dogma that T1DM is a T cell-mediated disease. This study underscores the importance of taking the entire immune response depicted in Figure 4 of this Primer into account when studying the aetiology and pathogenesis of T1DM.
Orban, T. et al . Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 37 , 1069–1075 (2014).
Gottlieb, P. A. et al . Failure to preserve β-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care 33 , 826–832 (2010).
Long, S. A. et al . Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61 , 2340–2348 (2012).
Agardh, C. D., Lynch, K. F., Palmer, M., Link, K. & Lernmark, Å. GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 52 , 1363–1368 (2009).
Ludvigsson, J. et al . GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. 359 , 1909–1920 (2008).
Ludvigsson, J. et al . GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366 , 433–442 (2012).
Wherrett, D. K. et al . Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378 , 319–327 (2011).
Haller, M. J. et al . Anti-thymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes 65 , 3765–3775 (2016).
Haller, M. J. et al . Autologous umbilical cord blood infusion for type 1 diabetes. Exp. Hematol. 36 , 710–715 (2008).
Bott, U., Muhlhauser, I., Overmann, H. & Berger, M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care 21 , 757–769 (1998).
Rubin, R. R. Diabetes and quality of life. Diabetes Spectr. 13 , 21–22 (2000).
Speight, J., Reaney, M. D. & Barnard, K. D. Not all roads lead to Rome — a review of quality of life measurement in adults with diabetes. Diabet. Med. 26 , 315–327 (2009).
Hilliard, M. E., Mann, K. A., Peugh, J. L. & Hood, K. K. How poorer quality of life in adolescence predicts subsequent type 1 diabetes management and control. Patient Educ. Couns. 91 , 120–125 (2013).
Hoey, H. et al . Good metabolic control is associated with better quality of life in 2,101 adolescents with type 1 diabetes. Diabetes Care 24 , 1923–1928 (2001).
Hood, K. K. et al . Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J. Adolesc. Health 55 , 498–504 (2014).
Laffel, L. M. et al . General quality of life in youth with type 1 diabetes: relationship to patient management and diabetes-specific family conflict. Diabetes Care 26 , 3067–3073 (2003).
Delamater, A. M. Psychological care of children and adolescents with diabetes. Pediatr. Diabetes 10 (Suppl. 12), 175–184 (2009).
Lohr, K. N. & Zebrack, B. J. Using patient-reported outcomes in clinical practice: challenges and opportunities. Qual. Life Res. 18 , 99–107 (2009).
Lernmark, Å. The streetlight effect — is there light at the end of the tunnel? Diabetes 64 , 1105–1107 (2015).
Rolandsson, O. & Palmer, J. P. Latent autoimmune diabetes in adults (LADA) is dead: long live autoimmune diabetes! Diabetologia 53 , 1250–1253 (2010).
Garg, S. K. et al . Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol. Ther. http://dx.doi.org/10.1089/dia.2016.0421 (2017).
Bally, L. et al . Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. http://dx.doi.org/10.1016/S2213-8587(17)30001-3 (2017).
de Wit, M. et al . Monitoring and discussing health related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabetes Care 31 , 1521–1526 (2008).
Little, R. R. & Rohlfing, C. L. The long and winding road to optimal HbA1c measurement. Clin. Chim. Acta 418 , 63–71 (2013).
Diabetes Prevention Trial — Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346 , 1685–1691 (2002).
Nanto-Salonen, K. et al . Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 372 , 1746–1755 (2008).
Gale, E. A., Bingley, P. J., Emmett, C. L. & Collier, T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363 , 925–931 (2004).
Download references
Acknowledgements
The authors were supported by the US NIH (grants K12DK097696 and R21DK106505 to B.J.A.; DK60987, DK60987, DK104216 and UL1TR001427 to D.A.S. and L.M.J.; and DK063861 to Å.L.), The Leona M. and Harry B. Helmsley Charitable Trust (grants 2015PG-T1D084 and 2016PG-T1D011 to B.J.A.) and the Swedish Research Council (Å.L., S.G. and A.R.).
Author information
Authors and affiliations.
Department of Clinical Sciences, Lund University, Skåne University Hospital, Jan Waldenströms gata 35, Malmö, 20502, Sweden
Anastasia Katsarou & Åke Lernmark
Institute of Medicine, Sahlgrenska University Hospital and University of Gothenburg, Gothenburg, Sweden
Soffia Gudbjörnsdottir & Araz Rawshani
Sweden National Diabetes Register, Centre of Registers, Gothenburg, Sweden
Department of Epidemiology, Colorado School of Public Health, Aurora, Colorado, USA
Dana Dabelea
Center for Regenerative Therapies Dresden, Technische Universität Dresden, Dresden, Germany
Ezio Bonifacio
Department of Pediatrics, Baylor College of Medicine Education at Texas Children's Hospital, Houston, Texas, USA
Barbara J. Anderson
Department of Pediatrics, University of Florida, Gainesville, Florida, USA
Laura M. Jacobsen & Desmond A. Schatz
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (A.K. and Å.L.); Epidemiology (A.K., Å.L. and D.D.); Mechanisms/pathophysiology (E.B.); Diagnosis, screening and prevention (A.K., Å.L., E.B. and D.D.); Management (D.A.S., L.M.J., S.G. and A.R.); Quality of life (B.J.A.); Outlook (Å.L.); Overview of Primer (Å.L.).
Corresponding author
Correspondence to Åke Lernmark .
Ethics declarations
Competing interests.
Å.L. is a member of the Scientific Advisory Board of Diamyd Medical, Stockholm, Sweden. All other authors declare no conflicts of interest.
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2, powerpoint slide for fig. 3, powerpoint slide for fig. 4, powerpoint slide for fig. 5, powerpoint slide for fig. 6, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Katsarou, A., Gudbjörnsdottir, S., Rawshani, A. et al. Type 1 diabetes mellitus. Nat Rev Dis Primers 3 , 17016 (2017). https://doi.org/10.1038/nrdp.2017.16
Download citation
Published : 30 March 2017
DOI : https://doi.org/10.1038/nrdp.2017.16
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Determinants of self-care among jordanian children with type 1 diabetes mellitus.
- Salam Hamdan
- Esra’ Taybeh
- Mervat M. Alsous
Journal of the Egyptian Public Health Association (2024)
Investigation of missense mutation-related type 1 diabetes mellitus through integrating genomic databases and bioinformatic approach
- Dyonisa Nasirochmi Pakha
- Ratih Dewi Yudhani
- Lalu Muhammad Irham
Genomics & Informatics (2024)
Construction and evaluation of sarcopenia risk prediction model for patients with diabetes: a study based on the China health and retirement longitudinal study (CHARLS)
- Mingrui Zou
- Zhenxing Shao
Diabetology & Metabolic Syndrome (2024)
Effectiveness of a standardized scenario in teaching the management of pediatric diabetic ketoacidosis (DKA) to residents: a simulation cross-sectional study
- Alice Monzani
- Elena Corti
- Ivana Rabbone
BMC Medical Education (2024)
Type 1 diabetes, its complications, and non-ischemic cardiomyopathy: a mendelian randomization study of European ancestry
- Yunyue Zhao
Cardiovascular Diabetology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
(A) The probability of developing diabetes in childhood stratified by the number of islet antibodies. In a study by Zeigler and colleagues, 16 13 377 children were identified as at risk in the newborn or infant period on the basis of high-risk HLA genotypes or having a relative with type 1 diabetes, or both, and were followed-up regularly. The numbers at risk are the number of children ...
Although research into type 1 diabetes prevention and disease modification continues to produce encouraging data, none of the approaches discussed above appears sufficiently effective alone in preventing or managing type 1 diabetes. Future endeavours will, therefore, require a novel focus, leveraging prior experience with regard to the ...
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. The loss of insulin leads to the ...
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to ...
Type 1 diabetes is a persistent autoimmune disease marked by insulin insufficiency and consequent hyperglycemia [4].It is caused by autoimmune beta-cell destruction in the pancreas, which leads to total insulin deficiency [5].Type 1 diabetes affects several people globally and needs cautious supervision to avoid grave complications, which include cardiovascular and renal disease, loss of ...
Type 1 diabetes (T1D) is a condition characterized by the immune-mediated destruction of insulin-producing pancreatic β-cells, leading to absolute insulin deficiency. The metabolic, genetic, and immunogenetic characteristics of T1D are heterogeneous, with age-related differences necessitating a personalized approach for each individual. Underlying genetic risk is present in many individuals ...
The burden of type 1 diabetes in 2021 is vast and is expected to increase rapidly, especially in resource-limited countries. Most incident and prevalent cases are adults. The substantial missing prevalence highlights the premature mortality of type 1 diabetes and an opportunity to save and extend lives of people with type 1 diabetes. Our new model, which will be made publicly available as the ...
Type 1 Diabetes (T1D) is a potentially life-threatening multifactorial autoimmune disorder characterized by T-cell-mediated destruction of pancreatic β cells, resulting in a deficiency of insulin synthesis and secretion [].The incidence of T1D has been rising globally since the 1950s, with an average annual increase of 3-4% over the past three decades [].
Type 1 diabetes (T1D) is an autoimmune disease characterized by loss of insulin producing beta cells and reliance on exogenous insulin for survival. T1D is one of the most common chronic diseases in childhood and the incidence is increasing, especially in children less than 5 years of age.
Type 1 diabetes mellitus (T1DM), also known as autoimmune diabetes, is a chronic disease characterized by insulin deficiency due to pancreatic β-cell loss and leads to hyperglycaemia. Although ...