An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


INTERMITTENT FASTING AND HUMAN METABOLIC HEALTH
Ruth e patterson , phd, gail a laughlin , phd, dorothy d sears , phd, andrea z lacroix , phd, catherine marinac , ba, linda c gallo , phd, sheri j hartman , phd, loki natarajan , phd, carolyn m senger , md, maría elena martínez , phd, adriana villaseñor , phd.
- Author information
- Article notes
- Copyright and License information
Corresponding Author/Reprint Contact: Ruth E. Patterson, Ph.D., Program Leader, Cancer Prevention and Control Program, Moores Cancer Center, University of California, San Diego, 3855 Health Sciences Drive #0901, La Jolla, CA 92093, Phone: (858) 534-2563, Fax (858) 822-2399, [email protected]
Author Contact Information
Ruth E. Patterson, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, 3855 Health Sciences Drive, La Jolla, CA 92093, (858) 534-2563, [email protected]
Gail A. Laughlin, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, (858) 822-2416, [email protected]
Dorothy D. Sears, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Medicine, Division of Endocrinology and Metabolism, University of California, La Jolla, California, USA, (858) 534-8898, [email protected]
Andrea Z. LaCroix, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, (858) 822-0627, [email protected]
Catherine Marinac, BA, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Graduate School of Public Health, San Diego State University, San Diego, CA, USA, [email protected]
Linda C. Gallo, PhD, Department of Psychology, San Diego State University, San Diego, CA, USA, (619) 594-4833, [email protected]
Sheri J. Hartman, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, [email protected] , (858) 534-9235
Loki Natarajan, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, (858) 822-4763, [email protected]
Carolyn M. Senger, MD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, [email protected]
María Elena Martínez, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, (858) 822-3638, [email protected]
Adriana Villaseñor, PhD, Moores UCSD Cancer Center, University of California, San Diego, La Jolla, CA, Department of Family & Preventive Medicine, University of California, San Diego, La Jolla, CA, USA, (858) 822-6827, [email protected]
Issue date 2015 Aug.
Key words/phrases: Diet, Intermittent Fasting, Metabolism, Obesity
INTRODUCTION
Periods of voluntary abstinence from food and drink (i.e., intermittent fasting) has been practiced since earliest antiquity by peoples around the globe. Books on ethnology and religion describe a remarkable variety of fasting forms and practices. 1 Renewed interest in fasting regimens is evidenced by a plethora of popular press publications and diet recommendations. For example, in 2013, Mosley and Spencer published a best-selling book titled “The Fast Diet,” which touts the benefits of restricting energy intake severely for two days a week while eating normally the rest of the week. 2 Dozens of books promote various fasting dietary patterns and the web offers hundreds of fasting-related sites. However, scientific evidence for the health benefits of intermittent fasting in humans is often extrapolated from animal studies, based on observational data on religious fasting (particularly Ramadan), or derived from experimental studies with modest sample sizes.
The overall objective of this paper is to provide an overview of intermittent fasting regimens ( Table 1 ) and summarize the evidence on the health benefits of intermittent fasting with a focus on human intervention studies. Because much of the data on intermittent fasting is from research in animal models, we briefly summarize key rodent studies and reviews. Health outcomes of interest are changes in weight and metabolic parameters associated with type 2 diabetes, cardiovascular disease, and cancer. We also present an overview of the major mechanisms hypothesized to link fasting regimens with human health: (1) circadian biology, (2) the gastrointestinal microbiota, and (3) modifiable lifestyle behaviors such as diet, activity, and sleep. Finally, we present conclusions regarding the evidence-base for intermittent fasting as an intervention for improving human health and propose a research agenda.
Types of intermittent fasting regimens that are hypothesized to impact health outcomes
This paper provides a uniquely broad synthesis of the scientific evidence linking intermittent fasting with human health and a framework for future research on this topic.
As noted above, we present a brief background of this considerable literature on intermittent fasting in animal models to provide context to the translational research that has been completed in humans. For human studies, we focus on findings from interventions that examined alternate day fasting, modified fasting regimens, and time-restricted feeding ( Table 1 ). A Medline search in PubMed was performed using the terms “intermittent fasting”, “fasting”, “time-restricted feeding” and “food timing”. In addition, we culled relevant papers from the reference list of research papers as well as reviews of fasting regimens. 3 , 4 Inclusion criteria for human studies were: (1) randomized controlled trials and nonrandomized trials, (2) adult male or female participants, and (3) endpoints of changes in body weight or biomarkers of risk of diabetes, cardiovascular disease or cancer. This is not a formal review or a meta-analysis: these studies cannot be combined because they are markedly dissimilar with regards to the intervention, the comparison group (or lack thereof), sample composition, study design, and intervention duration. Intermittent fasting performed as a religious practice (e.g., Ramadan) is reviewed separately and with less detail because these eating patterns are not motivated by health reasons and have generally been studied using observational study designs.
INTERMITTENT FASTING: HUMAN INTERVENTION TRIALS
This summary emphasizes findings from intervention trials ( Table 2 ) that provide evidence for evaluating the influence of intermittent fasting on human health.
Human intervention studies testing the impacts of intermittent fasting regimens on weight and metabolic biomarkers associated with risk of diabetes, cardiovascular disease, and cancer.
Abbreviations: ↓ denotes a statistically significant decrease (p<0.05); ↑ denotes a statistically significant increase (p<0.05); NS = not statistically significant (p≥0.05); BDNF = brain-derived neurotrophic factor; CRP = C-reactive protein; F = female; HbA1C = hemoglobin A1C; LDL; low-density lipoproteins; HDL = high-density lipoproteins; M = male; TG = triacylglycerides; TNF-α = tumor necrosis factor alpha.
No significant differences between fasting groups.
Alternate Day Fasting
Alternate day fasting involves “fasting days” in which no energy-containing foods or beverages are consumed alternating with days where foods and beverages are consumed ad libitum. In 2007, Varady and Hellerstein reviewed alternate day fasting studies in animals and concluded that this fasting regimen was as effective as simple caloric restriction in decreasing fasting insulin and glucose concentrations. 3 Alternate day fasting in animals also reduced total plasma cholesterol and triglyceride (TG) concentrations, and had beneficial effects on cancer risk factors such as cell proliferation.
To our knowledge, three intervention studies have explored the metabolic effects of alternate day fasting ( Table 2 ). 5 – 7 Sample sizes were modest and ranged from 8 to 30 normal weight adults. No information was provided about physical activity levels of these participants. Two of three studies reported significant weight loss, although we question the clinical relevance of weight loss in a 1-day study. 7 In the 22 day study of alternate date fasting, participants experienced a mean of 2.5% weight loss (p<0.001). 6 All studies found a significant decrease in at least one glucoregulatory marker. One study examined lipids with mixed results: improvements in high-density lipoprotein (HDL) cholesterol and TGs, but increased low-density lipoprotein (LDL) cholesterol. One of two studies found significant improvements in inflammatory markers.
Although limited, these data suggest that alternate day fasting regimens can result in modest weight loss. These data also show some positive impacts on metabolic parameters, even though these studies enrolled normal-weight adults who were unlikely to show substantial improvements in metabolic risk factors. However, Heilbronn et al 6 noted that self-reported hunger on fasting days was considerable and did not decrease over time, suggesting that alternate day fasting may not be a feasible public health intervention.
Modified Fasting Regimens
Modified fasting regimens generally allow for the consumption of 20–25% of energy needs on regularly scheduled “fasting” days. In these studies, the term fasting describes periods of severely limited energy intake rather than no energy intake. This regimen is the basis for the popular 5:2 diet, which involves energy restriction for 2 non-consecutive days a week and usual eating the other 5 days. 2
Varady et al has investigated the impacts of modified alternate-day fasting in mice. In a trial comparing 85% energy restriction on alternate fasting days to ad libitum chow, the energy restricted condition resulted in decreased visceral fat, leptin and resistin and increases in adiponectin. 8 Similar studies conducted by this research group also found that these fasting regimens in mice appear to reduce adipocyte size, cell proliferation, and levels of insulin-like growth factor-1. 9 – 11
As shown in Table 2 , we identified 8 trials of modified fasting in humans 12 – 19 Study sample sizes ranged from 10 to 107 adults, all of whom were overweight or obese. The duration of these fasting interventions ranged from 8 weeks to six months. Of the 8 studies, only 1 instituted weekly exercise goals. 12 Overall, six of eight studies (75%) reported statistically significant weight loss, which ranged from 3.2% in comparison to a control group 16 over a 12 week period to 8.0% in a one-arm trial over an 8 week period. 13 Two of five studies found significant decreases in fasting insulin, but none found reductions in fasting glucose. Three of the eight studies found significant improvements in lipids. Two of five studies found significant improvements in inflammatory markers including c-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), adiponectin, leptin, and brain-derived neutrotophic factor (BDNF). Half of these studies assessed some aspect of mood or other behavioral side effects in response to the fasting regimen. 13 , 15 , 18 , 19 In general, these studies reported that a small number (generally < 15%) of participants reported negative side effects, such as feeling cold, irritable, low energy, or hungry. However, there were mean improvements in mood including reductions in tension, anger and fatigue and increases in self-confidence and positive mood.
Three of the eight trials summarized above compared modified fasting regimens to simple energy restriction. 12 , 15 , 18 As shown in Table 2 , the weight loss regimens were either 1200–1500 kcals 12 or 25% energy restriction per day. 15 , 18 One of these studies instituted weekly exercise goals. 12 In only one case did the fasting regimen result in significantly more weight loss than a standard weight loss diet (4.1%). 12 In two of these studies, there was significantly reduced insulin concentrations compared with energy restriction, but no other differences in biomarker concentrations. The 12-week, controlled weight loss trial found that modified fasting regimen combined with an exercise protocol produced significantly superior weight loss results (6.5%) compared to fasting alone (3.2%) or exercise alone (1.1%). 16
A number of reviews have compared the results of fasting regimens with continuous or daily energy restriction. 20 – 21 The most recent of these reviews (2014) found that intermittent fasting regimens demonstrated 3–8% reductions in body weight after 3–24 weeks in comparison to energy restriction, which demonstrated 4–14% reductions in weight after 6–24 weeks. 21 The authors also reported that these two weight loss strategies yielded comparable reductions in visceral fat mass, fasting insulin, and insulin resistance and no meaningful reductions in fasting glucose concentrations.
Results from these intervention trials of modified fasting regimens suggest that these eating patterns result in weight loss, with modest and mixed effects on glucoregulatory markers, lipids and inflammatory markers. However, there is little evidence to suggest that modified alternate day fasting produces superior weight loss or metabolic changes in comparison to standard energy restriction regimens.
Time-Restricted Feeding
Rothschild et al recently reviewed the animal literature on time-restricted feeding. Twelve studies were identified with daily fasting intervals ranging from 12 to 20 hours, in numerous mouse models, with variability in coordination with light/dark phases and composition of chow. 4 In spite of the heterogeneity of these studies, the authors concluded that in mice, time-restricted feeding was associated with reductions in body weight, total cholesterol, TGs, glucose, insulin, interleukin-6 (IL-6), and TNF-α; as well as improvements in insulin sensitivity. It is notable that these health outcomes occurred despite variable effects of intermittent fasting on weight loss.
Research in animals highlights the potential importance of synchronizing intermittent fasting regimens with daily circadian rhythms. Animals given unlimited access to a high-fat diet (HFD) eat frequently throughout the night and the day, disrupting their normal nocturnal feeding cycle. These ad libitum HFD-fed mice develop obesity, diabetes, and metabolic syndrome. However, it was unclear whether these diseases result from the high-fat diets, disruption of circadian rhythms, or both. Compared to ad libitum feeding, mice whose feeding was restricted to normal nocturnal eating times consumed equivalent energy but were protected from obesity, hyperinsulinemia, hepatic steatosis, and inflammation. 22
We were only able to identify two trials in humans that investigated the impacts of time-restricted feeding interventions that extend the duration of nighttime fasting. Neither trial prescribed or measured physical activity. Both of these cross-over studies found significant reductions in weight. In the study among 29 normal weight men (two weeks per study condition), a prescribed nighttime fasting interval of ≥11 hours resulted in a significant weight change difference between the intervention (−0.4 kg) and control (+0.6 kg) conditions, which translates into 1.3% weight loss. 23 No biomarkers were assessed. Another cross-over study compared the effect of consuming one afternoon meal per day for 8 weeks and reported 4.1% weight loss in comparison to an isocaloric diet consumed as three meals per day. 24 , 25 One meal per day was also associated with reductions in fasting glucose, and improvements in LDL- and HDL-cholesterol. While self-reported hunger was higher in the morning for those consuming 1 meal per day, this fasting regimen was considered acceptable because there were no mean changes in tension, depression, anger, vigor, fatigue, or confusion.
While clearly limited, results from these studies of time-restricted feeding are consistent with research in animals indicating that incorporation of regular fasting intervals and eating in accordance with normal daily circadian rhythms (i.e., daytime hours in humans) may be important for maintaining optimal metabolic function.
RELIGIOUS FASTING: OBSERVATIONAL RESEARCH
Many religions incorporate fasting for both spiritual and physical benefits. However, published research on these fasting regimens is almost entirely observational. Therefore we provide only an overview of these fasting regimens.
Ramadan Fasting
One of the five pillars of Islam is that healthy adult Muslims must fast from dawn to sunset during the holy month of Ramadan. In addition, fluid intake, cigarette smoking, and medications are forbidden. Depending on the season and the geographical location of the country, day fasting can vary from 11 to 22 hours. Islamic fasting during Ramadan does not require energy restriction; however, as intake of food and fluid becomes less frequent, changes in body weight may occur.
In 2012 meta-analysis of 35 studies examined weight change during Ramadan. Across these studies, participant age ranged from 18 to 58; just over half (52%) were conducted in males and females, 34% were in males only and 11% were in females only. 26 The authors of this review found statistically significant weight loss in 21 (62%) of these studies. 26 When pooled, the studies in this meta-analysis showed a 1.24 kg weight reduction (95% CI −1.60, −0.88 kg) over the month of Ramadan fasting. Across 16 follow-up studies, mean weight regain was 0.72 kg (95% CI 0.32, 1.13 kg) in the 2 weeks following Ramadan.
A 2013 meta-analysis of 30 cohort studies including healthy young men and women examined whether Ramadan fasting altered biomarkers in addition to weight. 27 The primary finding of this meta-analysis was that after Ramadan fasting, low-density lipoprotein and fasting blood glucose levels were decreased in both sex groups and also in the entire group compared to levels prior to Ramadan. 27 In females only, HDL cholesterol levels were significantly increased. In males, there was a significant decrease in weight, total cholesterol, and TGs. Some studies have reported that Ramadan fasts are associated with significantly lower concentrations of inflammatory markers such as CRP, IL-6, and TNF-α. 28 , 29
Ramadan is the most common form of time-restricted feeding and results in transitory weight loss, with mixed evidence for improvements in metabolic markers. However, this feeding pattern is in biologic opposition to human circadian rhythms (see below) and therefore unlikely to be pursued as a desirable weight loss intervention.
Other Religious Fasts
A study of 448 patients from hospitals in Utah found that Church of the Latter Day Saints followers who reported routine fasting (29%) exhibited significantly lower weight and lower fasting glucose as well as lower prevalence of diabetes (OR 0.41; 95% CI 0.17, 0.99) and coronary stenosis (0.42, 95% CI 0.21, 0.84). 30 Seventh-day Adventists emphasize a healthy diet and lifestyle as important expressions of their faith and live approximately 7.3 years longer than other white adults. This increase in life expectancy has been primarily attributed to healthful lifestyles including not smoking, eating a plant based diet, and regular exercise. 31 Seventh-day Adventists often consume their last of two daily meals in the afternoon, which results in a long nighttime fasting period that may be biologically important. While it is unknown what proportion of Seventh-day Adventists adhere to a 2 meals per day pattern, this meal pattern is typically chronic, and sometimes lifelong, which would allow sufficient time to achieve stable changes in physiology. 25 However, the relation of reduced meal frequency and prolonged nightly fasting with health among Adventists has not been studied. 32
MECHANISTIC FACTORS LINKING INTERMITTENT FASTING WITH HEALTH
Figure 1 illustrates how factors hypothesized to link intermittent fasting with health outcomes are related. Briefly, intermittent fasting regimens are hypothesized to influence metabolic regulation via effects on (1) circadian biology, (2) the gastrointestinal microbiota, and (3) modifiable lifestyle behaviors. Negative perturbations in these systems can produce a hostile metabolic milieu, which predisposes individuals to the development of obesity, diabetes, cardiovascular disease, and cancer. See recent review by Longo and Mattson for a detailed review of the molecular mechanisms potentially linking fasting with health outcomes. 33
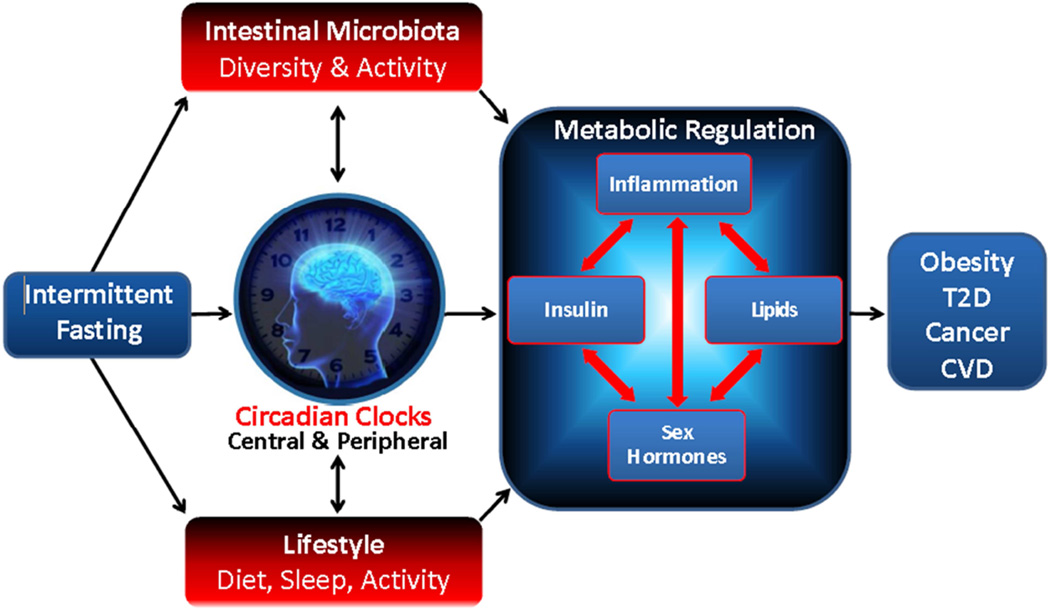
Association of intermittent fasting with intestinal microbiota, circadian clock, and other lifestyle factors hypothesized to result in metabolic regulation and downstream impacts on obesity, type 2 diabetes (T2D), cancer, and cardiovascular disease (CVD).
Circadian Biology
Intermittent fasting regimens that limit food consumption to daytime may leverage circadian biology to improve metabolic health. Organisms evolved to restrict their activity to the night or day by developing an endogenous circadian clock to ensure that physiological processes are performed at the optimal times. 34 Time of day plays a major role in the integration of metabolism and energetics as well as physiologic indices such as hormonal secretion patterns, physical coordination, and sleep ( Figure 2 ). 35 In mammals, the master biologic clock is located in the suprachiasmatic nuclei (SCN) of the hypothalamus and is entrained to light and dark stimuli. Similar clock oscillators have been found in peripheral tissues such as the liver; with feeding as the dominant timing cue (i.e., zeitgeber). It is hypothesized that desynchronization between the SCN master clock and peripheral circadian clocks disrupts energy balance 36 and leads to increased risk of chronic diseases. 37 It is hypothesized that some fasting regimens and time-restricted feeding impose a diurnal rhythm in food intake, resulting in improved oscillations in circadian clock gene expression that reprogram molecular mechanisms of energy metabolism and body weight regulation. 22 We refer interested readers to detailed reviews on the mechanisms underlying circadian biology. 34 – 39
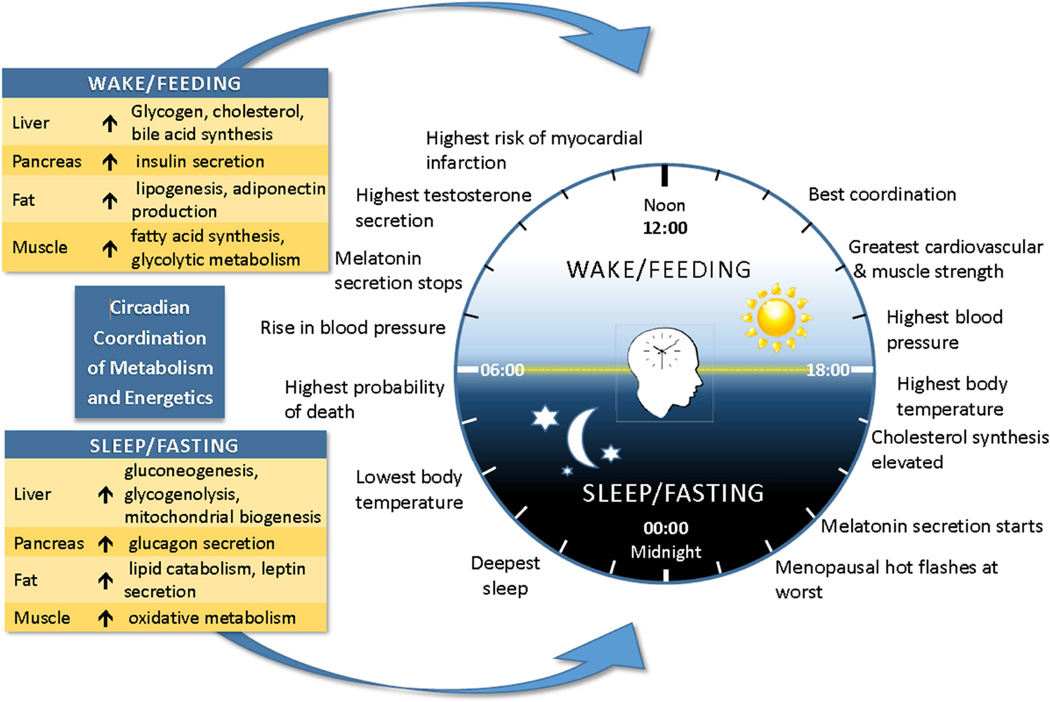
The human circadian rhythm regulates eating, sleeping, hormones, physiologic processes, and coordinates metabolism and energetics
The evidence that nutrient signals and meal-timing are circadian synchronizers is based largely on animal research. 38 , 39 However, in humans there is a large and robust literature indicating that shift work disrupts circadian rhythms and is associated with increased risk of obesity, diabetes, cardiovascular disease, and cancer (particularly breast cancer). 40 – 44 Similarly, data from trials and prospective cohorts support the hypothesis that consuming the majority of the day’s energys earlier in the day is associated with lower weight and improved health. 45 – 49
Gastrointestinal (Gut) Microbiota
Many functions of the gastrointestinal tract exhibit robust circadian or sleep-wake rhythms. For example, gastric emptying and blood flow are greater during the daytime than at night and metabolic responses to a glucose load are slower in the evening than in the morning. 50 Therefore, it is plausible that a chronically disturbed circadian profile may affect gastrointestinal function and impair metabolism and health. 51
Intermittent fasting may directly influence the gut microbiota, which is the complex, diverse, and vast microbial community that resides in the intestinal tract. Studies suggest that changes in composition and metabolic function of the gut microbiota in obese individuals may enable an “obese microbiota” to harvest more energy from the diet than a “lean microbiota” and thereby influence net energy absorption, expenditure, and storage. 52 – 54 In addition, obesity-related changes in gut microbiota can alter gut permeability and bacterial translocation to promote systemic inflammation 55 , a hallmark of obesity and obesity-related diseases. Finally, it is notable that a recent study has linked jet lag in mice and humans to abberrant microbiota diurnal fluctuations and dysbiosis that leads to glucose intolerance and obesity. 56
Modifiable Lifestyle Behaviors
Energy intake.
Metabolic unit studies of alternate and modified day fasting have documented decreased energy consumption. However, studies of fasting regimens in free-living adults are dependent on self-reported energy intake, which correlates poorly with objective markers of energy intake. 57 Weight change offers an indirect assessment of the impact of intermittent fasting on energy intake and as shown in Table 2 , statistically significant weight reduction was observed in 85% of intermittent fasting trials. Most fasting regimens reduce the total number of hours available for eating and thereby may reduce overall energy intake and risk of obesity. In addition, research in shift and night workers has demonstrated alterations in appetite-regulating hormones (leptin, ghrelin, xenin) that may lead to increases in total energy intake. 58 – 60
Energy Expenditure
Animal studies indicate that the circadian clock regulates locomotion. Mice on a time-restricted, isocaloric feeding regimen have shown improved muscle coordination and increased activity and energy expenditure toward the end of the feeding period. 22 However, data in humans is sparse or non-existent as to whether intermittent fasting regimens impact energy expenditure among free living adults.
Numerous observational studies have reported that nighttime eating is associated with reduced sleep duration and poor sleep quality, 61 , 62 which can lead to insulin resistance and increased risk of obesity, diabetes, cardiovascular disease, and cancer. 63 – 68 Specifically, eating meals at abnormal circadian times (i.e., late at night) is hypothesized to lead to circadian desynchronization 69 and subsequent disruption of normal sleep patterns. To our knowledge no studies have directly examined associations between intermittent fasting and sleep in free-living adults.
CONCLUSIONS
It is well known that in humans, even a single fasting interval (e.g., overnight) can reduce basal concentrations of metabolic biomarkers associated with chronic disease such as insulin and glucose. For example, patients are required to fast for 8–12 hours before blood draws to achieve steady-state fasting levels for many metabolic substrates. Therefore the important clinical and scientific question is whether adoption of a regular intermittent fasting regimen is a feasible and sustainable population-based strategy for promoting metabolic health. In addition, research is needed to test whether these regimens can complement or replace energy restriction and if so, whether they support long-term weight management. Below, we briefly summarize the major conclusions that can be drawn based on the current evidence.
Studies in rodents and other nocturnal mammals support the hypothesis that intermittent fasting and restricting the availability of chow to the normal nighttime feeding cycle improves metabolic profiles and reduces the risk of obesity, obesity-related conditions such as non-alcoholic fatty liver disease, and chronic diseases such as diabetes and cancer.
In healthy, normal weight, overweight, or obese adults, there is little evidence that intermittent fasting regimens are harmful physically or mentally (i.e., in terms of mood).
It appears that almost any intermittent fasting regimen can result in some weight loss. Among the 13 intervention trials included in this review, 11 (84.6%) reported statistically significant weight loss ranging from 1.3% in a cross-over trial with a 2 week intervention 23 to 8.0% in a 1-arm trial of 8 weeks duration. 13
Based on only 3 studies, alternate day fasting appears to results in weight loss as well as reductions in glucose and insulin concentrations. However, this pattern may not be practical because of intense hunger on fasting days.
Modified alternate day fasting regimens result in reduced weight, ranging from 3.2% in comparison to a control group 16 over a 12 week period to 8.0% in a one-arm trial over an 8 week period. 13 There was limited and mixed evidence for reductions in insulin concentrations, improvements in lipids or reductions in inflammatory factors.
Research to date has not demonstrated that alternate day fasting regimens produce superior weight loss in comparison to standard, continuous calorie restriction weight loss plans.
There are limited data from human studies to support the robust rodent data regarding the positive impacts of time-restricted feeding (i.e., eating patterns aligned with normal circadian rhythms) on weight or metabolic health.
There are considerable observational data on various forms of religious fasting, most of which suggests that these regimes result in transitory weight loss with mixed impacts on other biomarkers.
Data are lacking regarding the impacts of intermittent fasting on other health behaviors such as diet, sleep, and physical activity.
There are little or no published data linking intermittent fasting regimens with clinical outcomes such as diabetes, cardiovascular disease, cancer, or other chronic diseases such as Alzheimer’s.
A Research Agenda on Intermittent Fasting
Intermittent fasting regimens attempt to translate the positive effects of fasting regimens in rodents and other mammals into a practical eating pattern for reducing the risk of chronic disease in humans. Below we give suggestions for a future research agenda investigating intermittent fasting and metabolic health.
Modified fasting regimens appear to promote weight loss and may improve metabolic health. However, there are insufficient data to determine the optimal fasting regimen, including the length of the fasting interval, the number of “fasting” days per week, degree of energy restriction needed on fasting days, and recommendations for dietary behavior on non-fasting days.
Several lines of evidence support the hypothesis that eating patterns that reduce or eliminate nighttime eating and prolong nightly fasting intervals could result in sustained improvements in human health. While this hypothesis has not been tested in humans, support from animal research is striking and data from human time-restricted feeding studies are suggestive. Prolonged nightly fasting may be a simple, feasible, and potentially effective disease prevention strategy at the population level.
Large-scale randomized trials of intermittent fasting regimens in free-living adults are needed and should last for at least a year to see if behavioral and metabolic changes are sustainable and whether they have long term effects on biomarkers of aging and longevity. Future studies should incorporate objective measures of energy intake, sleep, and energy expenditure; assess numerous markers of disease risk; and enroll diverse populations who disproportionately suffer from obesity and related health maladies.
Current recommendations for weight loss frequently include advice to eat regular meals to avoid becoming hungry. Some guidelines also advise the consumption of regular snacks throughout the day. However, it is not clear that periods of fasting (i.e., hunger) necessarily lead to periods of over-eating. This overview suggests that intermittent fasting regimens may be a promising approach to lose weight and improve metabolic health for people who can tolerate intervals of not eating, or eating very little, for certain hours of the day or days of the week. If proven to be efficacious, these eating regimens may offer promising nonpharmacologic approaches to improving health at the population level with multiple public health benefits.
Acknowledgments
Funding Disclosure:
This work was supported (in part) by the National Cancer Institute Centers for Transdisciplinary Research on Energetics and Cancer (grant no. 1U54CA155435-01) and the National Cancer Institute, Comprehensive Partnerships to Advance Cancer Health Equity grants (U54CA132384 and U54CA132379). Dr. Hartman is supported by grant 1K07CA181323 from the National Cancer Institute, National Institutes of Health. Ms. Marinac is a recipient of a NCI-sponsored Ruth L. Kirschstein National Research Service Award (1F31CA183125-01A1). Dr. Villaseñor is supported by Diversity Research Supplement from the Continuing Umbrella of Research Experiences (CURE) training program, as part of the NCI Center to Reduce Cancer Health Disparities (CRCHD) (3U54CA155435-02S2).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no relevant interests to declare.
- 1. Brongers HA. Instruction and Interpretation: Studies in Hebrew Language, Palestinian Archaeology and Biblical Exegesis. Belgium: Brill Academic Pub.; 1997. [ Google Scholar ]
- 2. Mosley M, Spencer M. The FastDiet: Lose Weight, Stay Healthy, and Live Longer with the Simple Secret of Intermittent Fasting. Atria Books. 2013 [ Google Scholar ]
- 3. Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–318. doi: 10.1111/nure.12104. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Halberg N, Henriksen M, Soderhamn N, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol. 2005;99:2128–2136. doi: 10.1152/japplphysiol.00683.2005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Horne BD, Muhlestein JB, Lappe DL, et al. Randomized cross-over trial of short-term water-only fasting: Metabolic and cardiovascular consequences. Nutr Metab Cardiovasc Dis. 2013;23:1050–1057. doi: 10.1016/j.numecd.2012.09.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Varady KA, Hudak CS, Hellerstein MK. Modified alternate-day fasting and cardioprotection: relation to adipose tissue dynamics and dietary fat intake. Metabolism. 2009;58:803–811. doi: 10.1016/j.metabol.2009.01.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Varady KA, Roohk DJ, McEvoy-Hein BK, Gaylinn BD, Thorner MO, Hellerstein MK. Modified alternate-day fasting regimens reduce cell proliferation rates to a similar extent as daily calorie restriction in mice. FASEB J. 2008;22:2090–2096. doi: 10.1096/fj.07-098178. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Varady KA, Roohk DJ, Hellerstein MK. Dose effects of modified alternate-day fasting regimens on in vivo cell proliferation and plasma insulin-like growth factor-1 in mice. J Appl Physiol. 2007;103:547–551. doi: 10.1152/japplphysiol.00209.2007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Varady KA, Roohk DJ, Loe YC, McEvoy-Hein BK, Hellerstein MK. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J Lipid Res. 2007;48:2212–2219. doi: 10.1194/jlr.M700223-JLR200. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care. 1998;21:2–8. doi: 10.2337/diacare.21.1.2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes Suppl. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. 2013;2:1370–1379. doi: 10.1002/oby.20353. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12:4. doi: 10.1186/2251-6581-12-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Harvie MN, Wright C, Pegington M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534–1547. doi: 10.1017/S0007114513000792. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Varady KA, Bhutani S, Klempel MC, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:146. doi: 10.1186/1475-2891-12-146. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12:e593–e601. doi: 10.1111/j.1467-789X.2011.00873.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–311. doi: 10.1016/j.trsl.2014.05.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. LeCheminant JD, Christenson E, Bailey BW, Tucker LA. Restricting night-time eating reduces daily energy intake in healthy young men: a short-term cross-over study. Br J Nutr. 2013;110:2108–2113. doi: 10.1017/S0007114513001359. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metab Clin Exp. 2007;56:1729–1734. doi: 10.1016/j.metabol.2007.07.018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–988. doi: 10.1093/ajcn/85.4.981. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Sadeghirad B, Motaghipisheh S, Kolahdooz F, Zahedi MJ, Haghdoost AA. Islamic fasting and weight loss: a systematic review and meta-analysis. Public Health Nutr. 2014;17:3396–3406. doi: 10.1017/S1368980012005046. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Kul S, Savas E, Ozturk ZA, Karadag G. Does Ramadan Fasting Alter Body Weight and Blood Lipids and Fasting Blood Glucose in a Healthy Population? A Meta-analysis. J Relig Health. 2013;16:1217–1222. doi: 10.1007/s10943-013-9687-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Aksungar FB, Topkaya AE, Akyildiz M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann Nutr Metab. 2007;51:88–95. doi: 10.1159/000100954. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Faris MA, Kacimi S, Al-Kurd RA, et al. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32:947–955. doi: 10.1016/j.nutres.2012.06.021. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Horne BD, May HT, Anderson JL, et al. Usefulness of routine periodic fasting to lower risk of coronary artery disease in patients undergoing coronary angiography. Am J Cardiol. 2008;102:814–819. doi: 10.1016/j.amjcard.2008.05.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Fraser GE, Shavlik DJ. Ten years of life: Is it a matter of choice? Arch Intern Med. 2001;161(13):1645–1652. doi: 10.1001/archinte.161.13.1645. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Kelly CJ. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;86:1254–1255. doi: 10.1093/ajcn/86.4.1254. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging. 2010;2:7–27. doi: 10.18632/aging.100116. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Challet E. Circadian clocks, food intake, and metabolism. Prog Mol Biol Transl Sci. 2013;119:105–135. doi: 10.1016/B978-0-12-396971-2.00005-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Sensi S, Pace Palitti V, Guagnano MT. Chronobiology in endocrinology. Ann Ist Super Sanita. 1993;29:613–631. [ PubMed ] [ Google Scholar ]
- 40. Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lance Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Stevens RG, Blask DE, Brainard GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–1362. doi: 10.1289/ehp.10200. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Cause Control. 2001;12:279–287. doi: 10.1023/a:1011237000609. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Grundy A, Richardson H, Burstyn I, et al. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013;70:831–838. doi: 10.1136/oemed-2013-101482. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18:1249–1260. doi: 10.2119/molmed.2012.00077. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Jakubowicz D, Barnea M, Wainstein J, Froy O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21:2504–2512. doi: 10.1002/oby.20460. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Cahill LE, Chiuve SE, Mekary RA, et al. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;128:337–343. doi: 10.1161/CIRCULATIONAHA.113.001474. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Vander Wal JS. Night eating syndrome: a critical review of the literature. Clin Psychol Rev. 2012 Feb;32:49–59. doi: 10.1016/j.cpr.2011.11.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sciences. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Bo S, Musso G, Beccuti G, Fadda M, Fedele D, Gambino R, Gentile L, Durazzo M, Ghigo E, Cassader M. Consuming more of daily caloric intake at dinner predisposes to obesity. A 6-year population-based prospective cohort study. PLoS One. 2014;24(9):e108467. doi: 10.1371/journal.pone.0108467. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Sanders SW, Moore JG. Gastrointestinal chronopharmacology: physiology, pharmacology and therapeutic implications. Pharmacol Ther. 1992;54:1–15. doi: 10.1016/0163-7258(92)90049-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Ekmekcioglu C, Touitou Y. Chronobiological aspects of food intake and metabolism and their relevance on energy balance and weight regulation. Obesity Reviews. 2011;12:14–25. doi: 10.1111/j.1467-789X.2010.00716.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Ridaura VK, Faith JJ, Rey FE, et al. Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science. 2013;341:10. doi: 10.1126/science.1241214. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspect Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Thaiss CA, Zeevi D, Levy M, Ailberman-Schapira GZ, Suez J, Tengeler AC, et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol. 2014;180:172–188. doi: 10.1093/aje/kwu116. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Schiavo-Cardozo D, Lima MM, Pareja JC, Geloneze B. Appetite-regulating hormones from the upper gut: disrupted control of xenin and ghrelin in night workers. Clin Endocrinol. 2013;79:807–811. doi: 10.1111/cen.12114. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Crispim CA, Waterhouse J, Damaso AR, et al. Hormonal appetite control is altered by shift work: a preliminary study. Metab Clin Exp. 2011;60:1726–1735. doi: 10.1016/j.metabol.2011.04.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Wirth MD, Burch J, Shivappa N, et al. Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. J Occup Environ Med. 2014;56:145–148. doi: 10.1097/JOM.0000000000000088. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Antelmi E, Vinai P, Pizza F, Marcatelli M, Speciale M, Provini F. Nocturnal eating is part of the clinical spectrum of restless legs syndrome and an underestimated risk factor for increased body mass index. SleepMed. 2014;15:168–172. doi: 10.1016/j.sleep.2013.08.796. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Yamaguchi M, Uemura H, Katsuura-Kamano S, et al. Relationship of dietary factors and habits with sleep-wake regularity. Asia Pac J Clin Nutr. 2013;22:457–465. doi: 10.6133/apjcn.2013.22.3.01. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among U.S. adults. Obesity. 2014;22:598–607. doi: 10.1002/oby.20558. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (778.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 01 March 2024
Diet and nutrition
The weight-loss-independent benefits of fasting
- Benjamin D. Horne ORCID: orcid.org/0000-0002-2656-0263 1 , 2 , 3
Nature Metabolism volume 6 , pages 613–614 ( 2024 ) Cite this article
2795 Accesses
346 Altmetric
Metrics details
- Systems biology
- Translational research
In this study in humans, the authors describe distinct phases of adaptions in the plasma proteome to seven days without food, and identify limited associations of protein changes with weight loss.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Patikorn, C. et al. JAMA Netw. Open 4 , e2139558 (2021).
Article PubMed PubMed Central Google Scholar
Koppold, D. et al. Preprint at SSRN , https://doi.org/10.2139/ssrn.4665168 (2023).
Jamshed, H. et al. JAMA Intern. Med. 182 , 953–962 (2022).
Lin, S. et al. Ann. Intern. Med. 176 , 885–895 (2023).
Article PubMed Google Scholar
Bartholomew, C. L. et al. Eur. Heart J. Open 1 , oeab026 (2021).
Sutton, E. F. et al. Cell Metab. 27 , 1212–1221.e3 (2018).
Article CAS PubMed PubMed Central Google Scholar
Deru, L. S. et al. Med. Sci. Sports Exerc. 53 , 1987–1998 (2021).
Article CAS PubMed Google Scholar
Horne, B. D. et al. Nutr. Metab. Cardiovasc. Dis. 23 , 1050–1057 (2013).
Kamel, S. K., Lin, S. H., Cheema-Dhadli, S., Marliss, E. B. & Halperin, M. L. Kidney Int. 53 , 531–539 (1998).
Spark, R. F., Arky, R. A., Boulter, P. R., Saudek, C. D. & O’Brian, J. T. N. Engl. J. Med. 292 , 1335–1340 (1975).
DiNicolantonio, J. J. & McCarty, M. Open Heart 6 , e001028 (2019).
Mehrabani, S., Bagherniya, M., Askari, G., Read, M. I. & Sahebkar, A. J. Cachexia Sarcopenia Muscle 11 , 1447–1458 (2020).
Han, K. et al. Nat. Metab. 3 , 318–326 (2021).
Maifeld, A. et al. Nat. Commun. 12 , 1970 (2021).
Pietzner, M. et al. Nat. Metab. https://doi.org/10.1038/s42255-024-01008-9 (2024).
Download references
Author information
Authors and affiliations.
Intermountain Medical Center Heart Institute, Salt Lake City, UT, USA
Benjamin D. Horne
Division of Cardiovascular Medicine, Department of Medicine, Stanford University, Stanford, CA, USA
Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Benjamin D. Horne .

Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Horne, B.D. The weight-loss-independent benefits of fasting. Nat Metab 6 , 613–614 (2024). https://doi.org/10.1038/s42255-024-01012-z
Download citation
Published : 01 March 2024
Issue Date : April 2024
DOI : https://doi.org/10.1038/s42255-024-01012-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Sustainability
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Study reveals the benefits and downside of fasting
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Low-calorie diets and intermittent fasting have been shown to have numerous health benefits: They can delay the onset of some age-related diseases and lengthen lifespan, not only in humans but many other organisms.
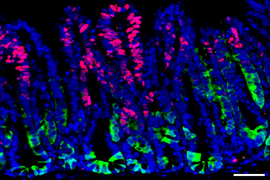
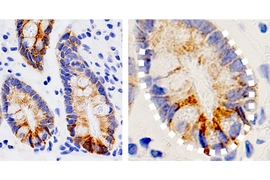
Many complex mechanisms underlie this phenomenon. Previous work from MIT has shown that one way fasting exerts its beneficial effects is by boosting the regenerative abilities of intestinal stem cells, which helps the intestine recover from injuries or inflammation.
In a study of mice, MIT researchers have now identified the pathway that enables this enhanced regeneration, which is activated once the mice begin “refeeding” after the fast. They also found a downside to this regeneration: When cancerous mutations occurred during the regenerative period, the mice were more likely to develop early-stage intestinal tumors.
“Having more stem cell activity is good for regeneration, but too much of a good thing over time can have less favorable consequences,” says Omer Yilmaz, an MIT associate professor of biology, a member of MIT’s Koch Institute for Integrative Cancer Research, and the senior author of the new study.
Yilmaz adds that further studies are needed before forming any conclusion as to whether fasting has a similar effect in humans.
“We still have a lot to learn, but it is interesting that being in either the state of fasting or refeeding when exposure to mutagen occurs can have a profound impact on the likelihood of developing a cancer in these well-defined mouse models,” he says.
MIT postdocs Shinya Imada and Saleh Khawaled are the lead authors of the paper, which appears today in Nature .
Driving regeneration
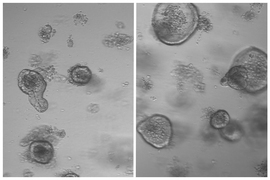
For several years, Yilmaz’s lab has been investigating how fasting and low-calorie diets affect intestinal health. In a 2018 study , his team reported that during a fast, intestinal stem cells begin to use lipids as an energy source, instead of carbohydrates. They also showed that fasting led to a significant boost in stem cells’ regenerative ability.
However, unanswered questions remained: How does fasting trigger this boost in regenerative ability, and when does the regeneration begin?
“Since that paper, we’ve really been focused on understanding what is it about fasting that drives regeneration,” Yilmaz says. “Is it fasting itself that’s driving regeneration, or eating after the fast?”
In their new study, the researchers found that stem cell regeneration is suppressed during fasting but then surges during the refeeding period. The researchers followed three groups of mice — one that fasted for 24 hours, another one that fasted for 24 hours and then was allowed to eat whatever they wanted during a 24-hour refeeding period, and a control group that ate whatever they wanted throughout the experiment.
The researchers analyzed intestinal stem cells’ ability to proliferate at different time points and found that the stem cells showed the highest levels of proliferation at the end of the 24-hour refeeding period. These cells were also more proliferative than intestinal stem cells from mice that had not fasted at all.
“We think that fasting and refeeding represent two distinct states,” Imada says. “In the fasted state, the ability of cells to use lipids and fatty acids as an energy source enables them to survive when nutrients are low. And then it’s the postfast refeeding state that really drives the regeneration. When nutrients become available, these stem cells and progenitor cells activate programs that enable them to build cellular mass and repopulate the intestinal lining.”
Further studies revealed that these cells activate a cellular signaling pathway known as mTOR, which is involved in cell growth and metabolism. One of mTOR’s roles is to regulate the translation of messenger RNA into protein, so when it’s activated, cells produce more protein. This protein synthesis is essential for stem cells to proliferate.
The researchers showed that mTOR activation in these stem cells also led to production of large quantities of polyamines — small molecules that help cells to grow and divide.
“In the refed state, you’ve got more proliferation, and you need to build cellular mass. That requires more protein, to build new cells, and those stem cells go on to build more differentiated cells or specialized intestinal cell types that line the intestine,” Khawaled says.
Too much of a good thing
The researchers also found that when stem cells are in this highly regenerative state, they are more prone to become cancerous. Intestinal stem cells are among the most actively dividing cells in the body, as they help the lining of the intestine completely turn over every five to 10 days. Because they divide so frequently, these stem cells are the most common source of precancerous cells in the intestine.
In this study, the researchers discovered that if they turned on a cancer-causing gene in the mice during the refeeding stage, they were much more likely to develop precancerous polyps than if the gene was turned on during the fasting state. Cancer-linked mutations that occurred during the refeeding state were also much more likely to produce polyps than mutations that occurred in mice that did not undergo the cycle of fasting and refeeding.
“I want to emphasize that this was all done in mice, using very well-defined cancer mutations. In humans it’s going to be a much more complex state,” Yilmaz says. “But it does lead us to the following notion: Fasting is very healthy, but if you’re unlucky and you’re refeeding after a fasting, and you get exposed to a mutagen, like a charred steak or something, you might actually be increasing your chances of developing a lesion that can go on to give rise to cancer.”
Yilmaz also noted that the regenerative benefits of fasting could be significant for people who undergo radiation treatment, which can damage the intestinal lining, or other types of intestinal injury. His lab is now studying whether polyamine supplements could help to stimulate this kind of regeneration, without the need to fast.
“This fascinating study provides insights into the complex interplay between food consumption, stem cell biology, and cancer risk,” says Ophir Klein, a professor of medicine at the University of California at San Francisco and Cedars-Sinai Medical Center, who was not involved in the study. “Their work lays a foundation for testing polyamines as compounds that may augment intestinal repair after injuries, and it suggests that careful consideration is needed when planning diet-based strategies for regeneration to avoid increasing cancer risk.”
The research was funded, in part, by Pew-Stewart Scholars Program for Cancer Research award, the MIT Stem Cell Initiative, the Koch Institute Frontier Research Program via the Kathy and Curt Marble Cancer Research Fund, and the Bridge Project, a partnership between the Koch Institute for Integrative Cancer Research at MIT and the Dana-Farber/Harvard Cancer Center.
Share this news article on:
Press mentions, medical news today.
A new study led by researchers at MIT suggests that fasting and then refeeding stimulates cell regeneration in the intestines, reports Katharine Lang for Medical News Today . However, notes Lang, researchers also found that fasting “carries the risk of stimulating the formation of intestinal tumors.”
MIT researchers have discovered how fasting impacts the regenerative abilities of intestinal stem cells, reports Ed Cara for Gizmodo . “The major finding of our current study is that refeeding after fasting is a distinct state from fasting itself,” explain Prof. Ömer Yilmaz and postdocs Shinya Imada and Saleh Khawaled. “Post-fasting refeeding augments the ability of intestinal stem cells to, for example, repair the intestine after injury.”
Prof. Ömer Yilmaz and his colleagues have discovered the potential health benefits and consequences of fasting, reports Max Kozlov for Nature . “There is so much emphasis on fasting and how long to be fasting that we’ve kind of overlooked this whole other side of the equation: what is going on in the refed state,” says Yilmaz.
Previous item Next item
Related Links
- Omer Yilmaz
- Koch Institute
- Department of Biology
Related Topics
Related articles.
How early-stage cancer cells hide from the immune system
Study links certain metabolites to stem cell function in the intestine
Fasting boosts stem cells’ regenerative capacity

How diet influences colon cancer
More mit news.
The MIT Press releases report on the future of open access publishing and policy
Read full story →

A blueprint for better cancer immunotherapies

Improving health, one machine learning system at a time

To design better water filters, MIT engineers look to manta rays

Professor Emeritus James Harris, a scholar of Spanish language, dies at 92

New solar projects will grow renewable energy generation for four major campus buildings
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram

IMAGES
VIDEO
COMMENTS
This article introduces the effects of fasting on human lipid metabolism, glucose metabolism, protein metabolism, and neuroendocrine metabolism; demonstrates the metabolic conversion caused by fasting; and describes the effects of fasting on human psychological health, the relationship between mood regulation and glucose, and the emotional ...
Research Highlights: A study of over 20,000 adults found that those who followed an 8-hour time-restricted eating schedule, a type of intermittent fasting, had a 91% higher risk of death from cardiovascular disease.
Hundreds of animal studies and scores of human clinical trials have shown that intermittent fasting can lead to improvements in health conditions such as obesity, diabetes, cardiovascular disease, cancers and neurological disorders. The evidence is less clear for lifespan effects.
Intermittent fasting is an alternative and easily applicable dietary intervention for caloric restriction. Moreover, intermittent fasting has beneficial effects equivalent to those of caloric restriction in terms of body weight control, improvements in glucose homeostasis and lipid profiles, and anti-inflammatory effects.
New findings reveal that the body undergoes significant, systematic changes across multiple organs during prolonged periods of fasting. The results demonstrate evidence of health benefits...
Our findings suggest that IF may have beneficial effects on a range of health outcomes for adults with overweight or obesity, compared to CER or non-intervention diet. Specifically, IF may decreased WC, fat mass, LDL-C, TG, TC, fasting insulin, and SBP, while increasing HDL-C and FFM.
Here are eight health benefits of fasting — backed by science. 1. Promotes blood sugar control by reducing insulin resistance. Several studies have found that fasting may improve...
Because much of the data on intermittent fasting is from research in animal models, we briefly summarize key rodent studies and reviews. Health outcomes of interest are changes in weight and metabolic parameters associated with type 2 diabetes, cardiovascular disease, and cancer.
The cardiovascular, metabolic and other systemic and organ-level health effects of fasting are frequently described as the many profits of weight loss, because reduced weight — regardless of ...
Low-calorie diets and intermittent fasting have been shown to have numerous health benefits: They can delay the onset of some age-related diseases and lengthen lifespan, not only in humans but many other organisms.