- Introduction
- Conclusions
- Article Information
BMI indicates body mass index; SES, socioeconomic status.
a Variables smoking status, SES, drinking pattern, former drinker bias only, occasional drinker bias, median age, and gender were removed.
b Variables race, diet, exercise, BMI, country, follow-up year, publication year, and unhealthy people exclusion were removed.
eAppendix. Methodology of Meta-analysis on All-Cause Mortality and Alcohol Consumption
eReferences
eFigure 1. Flowchart of Systematic Search Process for Studies of Alcohol Consumption and Risk of All-Cause Mortality
eTable 1. Newly Included 20 Studies (194 Risk Estimates) of All-Cause Mortality and Consumption in 2015 to 2022
eFigure 2. Funnel Plot of Log-Relative Risk (In(RR)) of All-Cause Mortality Due to Alcohol Consumption Against Inverse of Standard Error of In(RR)
eFigure 3. Relative Risk (95% CI) of All-Cause Mortality Due to Any Alcohol Consumption Without Any Adjustment for Characteristics of New Studies Published between 2015 and 2022
eFigure 4. Unadjusted, Partially Adjusted, and Fully Adjusted Relative Risk (RR) of All-Cause Mortality for Drinkers (vs Nondrinkers), 1980 to 2022
eTable 2. Statistical Analysis of Unadjusted Mean Relative Risk (RR) of All-Cause Mortality for Different Categories of Drinkers for Testing Publication Bias and Heterogeneity of RR Estimates From Included Studies
eTable 3. Mean Relative Risk (RR) Estimates of All-Cause Mortality Due to Alcohol Consumption up to 2022 for Subgroups (Cohorts Recruited 50 Years of Age or Younger and Followed up to 60 Years of Age)
Data Sharing Statement
- Errors in Figure and Supplement JAMA Network Open Correction May 9, 2023

See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Digital Health
- Drug Development
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Sexual Health
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zhao J , Stockwell T , Naimi T , Churchill S , Clay J , Sherk A. Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses . JAMA Netw Open. 2023;6(3):e236185. doi:10.1001/jamanetworkopen.2023.6185
Manage citations:
© 2024
- Permissions
Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses
- 1 Canadian Institute for Substance Use Research, University of Victoria, Victoria, British Columbia, Canada
- 2 Department of Psychology, University of Portsmouth, Portsmouth, Hampshire, United Kingdom
- Correction Errors in Figure and Supplement JAMA Network Open
Question What is the association between mean daily alcohol intake and all-cause mortality?
Findings This systematic review and meta-analysis of 107 cohort studies involving more than 4.8 million participants found no significant reductions in risk of all-cause mortality for drinkers who drank less than 25 g of ethanol per day (about 2 Canadian standard drinks compared with lifetime nondrinkers) after adjustment for key study characteristics such as median age and sex of study cohorts. There was a significantly increased risk of all-cause mortality among female drinkers who drank 25 or more grams per day and among male drinkers who drank 45 or more grams per day.
Meaning Low-volume alcohol drinking was not associated with protection against death from all causes.
Importance A previous meta-analysis of the association between alcohol use and all-cause mortality found no statistically significant reductions in mortality risk at low levels of consumption compared with lifetime nondrinkers. However, the risk estimates may have been affected by the number and quality of studies then available, especially those for women and younger cohorts.
Objective To investigate the association between alcohol use and all-cause mortality, and how sources of bias may change results.
Data Sources A systematic search of PubMed and Web of Science was performed to identify studies published between January 1980 and July 2021.
Study Selection Cohort studies were identified by systematic review to facilitate comparisons of studies with and without some degree of controls for biases affecting distinctions between abstainers and drinkers. The review identified 107 studies of alcohol use and all-cause mortality published from 1980 to July 2021.
Data Extraction and Synthesis Mixed linear regression models were used to model relative risks, first pooled for all studies and then stratified by cohort median age (<56 vs ≥56 years) and sex (male vs female). Data were analyzed from September 2021 to August 2022.
Main Outcomes and Measures Relative risk estimates for the association between mean daily alcohol intake and all-cause mortality.
Results There were 724 risk estimates of all-cause mortality due to alcohol intake from the 107 cohort studies (4 838 825 participants and 425 564 deaths available) for the analysis. In models adjusting for potential confounding effects of sampling variation, former drinker bias, and other prespecified study-level quality criteria, the meta-analysis of all 107 included studies found no significantly reduced risk of all-cause mortality among occasional (>0 to <1.3 g of ethanol per day; relative risk [RR], 0.96; 95% CI, 0.86-1.06; P = .41) or low-volume drinkers (1.3-24.0 g per day; RR, 0.93; P = .07) compared with lifetime nondrinkers. In the fully adjusted model, there was a nonsignificantly increased risk of all-cause mortality among drinkers who drank 25 to 44 g per day (RR, 1.05; P = .28) and significantly increased risk for drinkers who drank 45 to 64 and 65 or more grams per day (RR, 1.19 and 1.35; P < .001). There were significantly larger risks of mortality among female drinkers compared with female lifetime nondrinkers (RR, 1.22; P = .03).
Conclusions and Relevance In this updated systematic review and meta-analysis, daily low or moderate alcohol intake was not significantly associated with all-cause mortality risk, while increased risk was evident at higher consumption levels, starting at lower levels for women than men.
The proposition that low-dose alcohol use protects against all-cause mortality in general populations continues to be controversial. 1 Observational studies tend to show that people classified as “moderate drinkers” have longer life expectancy and are less likely to die from heart disease than those classified as abstainers. 2 Systematic reviews and meta-analyses of this literature 3 confirm J-shaped risk curves (protective associations at low doses with increasing risk at higher doses). However, mounting evidence suggests these associations might be due to systematic biases that affect many studies. For example, light and moderate drinkers are systematically healthier than current abstainers on a range of health indicators unlikely to be associated with alcohol use eg, dental hygiene, exercise routines, diet, weight, income 4 ; lifetime abstainers may be systematically biased toward poorer health 5 ; studies fail to control for biases in the abstainer reference group, in particular failing to remove “sick quitters” or former drinkers, many of whom cut down or stop for health reasons 2 ; and most studies have nonrepresentative samples leading to an overrepresentation of older White men. Adjustment of cohort samples to make them more representative has been shown to eliminate apparent protective associations. 6 Mendelian randomization studies that control for the confounding effects of sociodemographic and environmental factors find no evidence of cardioprotection. 7
We published 2 previous systematic reviews and meta-analyses that investigated these hypotheses. The first of these focused on all-cause mortality, 8 finding negligible reductions in mortality risk with low-volume alcohol use when study-level controls were introduced for potential bias and confounding, such as the widespread practice of misclassifying former drinkers and/or current occasional drinkers as abstainers (ie, not restricting reference groups to lifetime abstainers). 8 Our alcohol and coronary heart disease (CHD) mortality meta-analysis of 45 cohort studies 9 found that CHD mortality risk differed widely by age ranges and sex of study populations. In particular, young cohorts followed up to old age did not show significant cardio-protection for low-volume use. Cardio-protection was only apparent among older cohorts that are more exposed to lifetime selection biases (ie, increasing numbers of “sick-quitters” in the abstainer reference groups and the disproportionate elimination of drinkers from the study sample who had died or were unwell).
The present study updates our earlier systematic review and meta-analysis for all-cause mortality and alcohol use, 8 including studies published up to July 2021 (ie, 6.5 years of additional publications). The study also investigated the risk of all-cause mortality for alcohol consumption according to (1) median ages of the study populations (younger than 56 years or 56 years and older), replicating the methods of Zhao et al 9 ; (2) the sex distribution of the study populations, and (3) studies of cohorts recruited before a median age of 51 years of age and followed up in health records until a median age of at least 60 years (ie, with stricter rules to further minimize lifetime selection biases). Because younger cohorts followed up to an age at which they may experience heart disease are less likely to be affected by lifetime selection biases, 9 we hypothesized that such studies would be less likely to show reduced mortality risks for low-volume drinkers. Finally, we reran the analyses using occasional drinkers (<1 drink per week) as the reference, for whom physiological health benefits are unlikely. Occasional drinkers are a more appropriate reference group, given evidence demonstrating that lifetime abstainers may be biased toward ill health. 10
The present study updates the systematic reviews and meta-analyses described above 8 by including studies published up to July 2021 to investigate whether the risk differed for subgroups. The study protocol was preregistered on the Open Science Framework. 11 Inclusion criteria, search strategy, study selection, data extraction, and statistical analytical methods of the study are summarized in later sections (see eAppendix in Supplement 1 for more details).
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline. 12 The review sought cohort studies of all-cause mortality and alcohol consumption. We identified all potentially relevant articles published up to July 31, 2021, regardless of language, by searching PubMed and Web of Science, through reference list cross-checking of previous meta-analyses (eFigure 1 in Supplement 1 ). There were 87 studies identified by Stockwell et al. 8 After inclusion of 20 new studies meeting inclusion criteria, there were a total of 107 cohort studies (eTable 1 in Supplement 1 ). 13 - 32
Three coders (J. Z., F. A., and J. C.) reviewed all eligible studies to extract and code data independently from all studies fulfilling the inclusion criteria. Data extracted included (1) outcome, all-cause mortality; (2) measures of alcohol consumption; (3) study characteristics, including cohort ages at recruitment and follow-up; (4) types of misclassification error of alcohol consumers and abstainers; (5) controlled variables in individual studies. Alcoholic drinks were converted into grams per day according to country-specific definitions if not otherwise defined. 33 , 34
We also assessed publication bias, heterogeneity, and confounding of covariates that might potentially affect the association of interest using several statistical approaches. 35 - 41 Relative risk (RR), including hazard ratios or rate ratios, were converted to natural log-transformed formats to deal with skewness. Publication bias was assessed through visual inspection of the funnel plot of log-RR of all-cause mortality due to alcohol consumption against the inverse standard error of log-RR 42 and Egger’s linear regression method. 36 We also plotted forest graphs of log-RR of all-cause mortality for any level of drinking to assess heterogeneity among studies. 42 The between-study heterogeneity of RRs were assessed using Cochran Q 37 and the I 2 statistic. 38 If heterogeneity was detected, mixed-effects models were used to obtain the summarized RR estimates. Mixed-effects regression analyses were performed in which drinking groups and control variables were treated as fixed-effects with a random study effect because of significant heterogeneity. 43
All analyses were weighted by the inverse of the estimated variance of the natural log relative risk. Variance was estimated from reported standard errors, confidence intervals, or number of deaths. The weights for each individual study were created using the inverse variance weight scheme and used in mixed regression analysis to get maximum precision for the main results of the meta-analysis. 42 In comparison with lifetime abstainers, the study estimated the mean RR of all-cause mortality for former drinkers (ie, now completely abstaining), current occasional (<9.1 g per week), low-volume (1.3-24.0 g per day), medium-volume (25.0-44.0 g per day), high-volume (45.0-64.0 g) and highest-volume drinkers (≥65.0 grams per day). The analyses adjusted for the potential confounding effects of study characteristics including the median age and sex distribution of study samples, drinker biases, country where a study was conducted, follow-up years and presence or absence of confounders. Analyses were also repeated using occasional drinkers as the reference group. We used t tests to calculate P values, and significance was set at .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute) and the SAS MIXED procedure was used to model the log-transformed RR. 44 Data were analyzed from September 2021 to August 2022.
There were 724 estimates of the risk relationship between level of alcohol consumption and all-cause mortality from 107 unique studies 13 - 32 , 45 - 131 , including 4 838 825 participants and 425 564 deaths available for the analysis. Table 1 describes the sample characteristics of the metadata. Of 39 studies 13 , 15 , 18 , 21 , 23 - 26 , 29 , 31 , 45 - 47 , 49 , 50 , 52 - 54 , 57 - 59 , 62 , 64 , 70 , 80 , 81 , 85 , 87 , 91 , 94 , 96 , 100 , 104 , 107 , 118 , 124 , 125 , 127 , 130 reporting RR estimates for men and women separately, 33 14 , 17 , 48 , 51 , 61 , 63 , 66 , 68 , 69 , 72 , 76 , 79 , 83 , 84 , 86 , 88 , 90 , 92 , 93 , 97 , 98 , 101 , 103 , 105 , 109 - 111 , 113 - 115 , 119 , 120 , 128 were for males only, 8 16 , 65 , 73 , 99 , 102 , 108 , 112 , 123 for females only, and 30 13 , 19 - 22 , 26 - 30 , 32 , 55 , 56 , 67 , 71 , 74 , 75 , 77 , 78 , 82 , 84 , 89 , 95 , 106 , 116 , 117 , 121 , 122 , 126 , 129 for both sexes. Twenty-one studies 13 , 17 , 19 , 21 , 22 , 26 , 27 , 45 - 58 (220 risk estimates) were free from abstainer bias (ie, had a reference group of strictly defined lifetime abstainers). There were 50 studies 14 - 16 , 18 , 20 , 23 - 25 , 29 , 59 - 99 (265 risk estimates) with both former and occasional drinker bias; 28 studies 28 , 30 - 32 , 100 - 122 , 130 (177 risk estimates) with only former drinker bias; and 8 studies 123 - 129 , 131 (62 risk estimates) with only occasional drinker bias.
Unadjusted mean RR estimates for most study subgroups categorized by methods/sample characteristics showed markedly or significantly higher RRs for alcohol consumers as a group vs abstainers. Exceptions were for studies with less than 10 years of follow-up and those with some form of abstainer bias ( Table 1 ). Bivariable analyses showed that mortality risks for alcohol consumers varied considerably according to other study characteristics, such as quality of the alcohol consumption measure, whether unhealthy individuals were excluded at baseline, and whether socioeconomic status was controlled for ( Table 1 ).
No evidence of publication bias was detected either by inspection of symmetry in the funnel plot of log-RR estimates and their inverse standard errors (eFigure 2 in Supplement 1 ) or by Egger linear regression analysis (eTable 2 in Supplement 1 , all P > .05 for each study group). Significant heterogeneity was observed across studies for all drinking categories confirmed by both the Q statistic ( Q 723 = 5314.80; P < .001) and I 2 estimates (all >85.87%). (See eFigure 3 in Supplement 1 for forest plot of unadjusted risk estimates of mortality risks for the 20 newly identified studies).
Pooled unadjusted estimates (724 observations) showed significantly higher risk for former drinkers (RR, 1.22; 95% CI, 1.11-1.33; P = .001) and significantly lower risk for low-volume drinkers (RR, 0.85; 95% CI, 0.81-0.88; P = .001) compared with abstainers as defined in the included studies ( Table 2 ; eFigure 4 in Supplement 1 ). In the fully adjusted model, mortality RR estimates increased for all drinking categories, becoming nonsignificant for low-volume drinkers (RR, 0.93; 95% CI, 0.85-1.01; P = .07), occasional drinkers (>0 to <1.3 g of ethanol per day; RR, 0.96; 95% CI, 0.86-1.06; P = .41), and drinkers who drank 25 to 44 g per day (RR, 1.05; 95% CI, 0.96-1.14; P = .28). There was a significantly increased risk among drinkers who drank 45 to 64 g per day (RR, 1.19; 95% CI, 1.07-1.32; P < .001) and 65 or more grams (RR, 1.35; 95% CI, 1.23-1.47; P < .001). The Figure shows the changes in RR estimates for low-volume drinkers when removing each covariate from the fully adjusted model. In most cases, removing study-level covariates tended to yield lower risk estimates from alcohol use.
Table 2 presents the RR estimates when occasional drinkers were the reference group. In fully adjusted models, higher though nonsignificant mortality risks were observed for both abstainers and medium-volume drinkers (RR, 1.04; 95% CI, 0.94-1.16; P = .44 and RR, 1.09; 95% CI, 0.96-1.25; P = .19, respectively). There were significantly elevated risks for both high and higher volume drinkers (RR, 1.24; 95% CI, 1.07-1.44; P = .004 and RR, 1.41; 95% CI, 1.23-1.61; . P = 001, respectively).
As hypothesized, there was a significant interaction between cohort age and mortality risk ( P = .02; F 601 = 2.93) and so RR estimates for drinkers were estimated in analyses stratified by median age of the study populations at enrollment ( Table 3 ). In unadjusted and partially adjusted analyses, older cohorts displayed larger reductions in mortality risk associated with low-volume consumption than younger cohorts. However, in fully adjusted analyses with multiple covariates included for study characteristics, these differences disappeared. Younger cohorts also displayed greater mortality risks than older cohorts at higher consumption levels. Among studies in which participants were recruited at age 50 years or younger and followed up to age 60 years (ie, there was likely reduced risk of lifetime selection bias) higher RR estimates were observed for all drinking groups vs lifetime abstainers. These differences were significant in all drinking groups except low-volume drinkers (eTable 3 in Supplement 1 ).
Across all levels of alcohol consumption, female drinkers had a higher RR of all-cause mortality than males ( P for interaction = .001). As can be seen in Table 4 , all female drinkers had a significantly increased mortality risk compared with female lifetime nondrinkers (RR, 1.22; 95% CI, 1.02-1.46; P = .03). Compared with lifetime abstainers, there was significantly increased risk of all-cause mortality among male drinkers who drank 45 to 64 g per day (RR, 1.15; 95% CI, 1.03-1.28; P = .01) and drank 65 or more (RR, 1.34; 95% CI, 1.23-1.47; P < .001), and among female drinkers who drank 25 to 44 g per day (RR, 1.21; 95% CI, 1.08-1.36; P < .01), 45 to 64 g (RR, 1.34; 95% CI, 1.11-1.63; P < .01) and 65 or more grams (RR, 1.61; 95% CI, 1.44-1.80; P = .001).
In fully adjusted, prespecified models that accounted for effects of sampling, between-study variation, and potential confounding from former drinker bias and other study-level covariates, our meta-analysis of 107 studies found (1) no significant protective associations of occasional or low-volume drinking (moderate drinking) with all-cause mortality; and (2) an increased risk of all-cause mortality for drinkers who drank 25 g or more and a significantly increased risk when drinking 45 g or more per day.
Several meta-analytic strategies were used to explore the role of abstainer reference group biases caused by drinker misclassification errors and also the potential confounding effects of other study-level quality covariates in studies. 2 Drinker misclassification errors were common. Of 107 studies identified, 86 included former drinkers and/or occasional drinkers in the abstainer reference group, and only 21 were free of both these abstainer biases. The importance of controlling for former drinker bias/misclassification is highlighted once more in our results which are consistent with prior studies showing that former drinkers have significantly elevated mortality risks compared with lifetime abstainers.
In addition to presenting our fully adjusted models, a strength of the study was the examination of the differences in relative risks according to unadjusted and partially adjusted models, including the effect of removing individual covariates from the fully adjusted model. We found evidence that abstainer biases and other study characteristics changed the shape of the risk relationship between mortality and rising alcohol consumption, and that most study-level controls increased the observed risks from alcohol, or attenuated protective associations at low levels of consumption such that they were no longer significant. The reduced RR estimates for occasional or moderate drinkers observed without adjustment may be due to the misclassification of former and occasional drinkers into the reference group, a possibility which is more likely to have occurred in studies of older cohorts which use current abstainers as the reference group. This study also demonstrates the degree to which observed associations between consumption and mortality are highly dependent on the modeling strategy used and the degree to which efforts are made to minimize confounding and other threats to validity.
It also examined risk estimates when using occasional drinkers rather than lifetime abstainers as the reference group. The occasional drinker reference group avoids the issue of former drinker misclassification that can affect the abstainer reference group, and may reduce confounding to the extent that occasional drinkers are more like low-volume drinkers than are lifetime abstainers. 2 , 8 , 132 In the unadjusted and partially adjusted analyses, using occasional drinkers as the reference group resulted in nonsignificant protective associations and lower point estimates for low-volume drinkers compared with significant protective associations and higher point estimates when using lifetime nondrinkers as the reference group. In the fully adjusted models, there were nonsignificant protective associations for low-volume drinkers whether using lifetime abstainers or occasional drinkers as the reference group, though this was only a RR of 0.97 for the latter.
Across all studies, there were few differences in risk for studies when stratified by median age of enrollment above or below age 56 years in the fully adjusted analyses. However, in the subset of studies who enrolled participants aged 50 years or younger who were followed for at least 10 years, occasional drinkers and medium-volume drinkers had significantly increased risk of mortality and substantially higher risk estimates for high- and higher-volume consumption compared with results from all studies. This is consistent with our previous meta-analysis for CHD, 9 in which younger cohorts followed up to older age did not show a significantly beneficial association of low-volume consumption, while older cohorts, with more opportunity for lifetime selection bias, showed marked, significant protective associations.
Our study also found sex differences in the risk of all-cause mortality. A larger risk of all-cause mortality for women than men was observed when drinking 25 or more grams per day, including a significant increase in risk for medium-level consumption for women that was not observed for men. However, mortality risk for mean consumption up to 25 g per day were very similar for both sexes.
A number of limitations need to be acknowledged. A major limitation involves imperfect measurement of alcohol consumption in most included studies, and the fact that consumption in many studies was assessed at only 1 point in time. Self-reported alcohol consumption is underreported in most epidemiological studies 133 , 134 and even the classification of drinkers as lifetime abstainers can be unreliable, with several studies in developed countries finding that the majority of self-reported lifetime abstainers are in fact former drinkers. 135 , 136 If this is the case, the risks of various levels of alcohol consumption relative to presumed lifetime abstainers are underestimates. Merely removing former drinkers from analyses may bias studies in favor of drinkers, since former drinkers may be unhealthy, and should rightly be reallocated to drinking groups according to their history. However, this has only been explored in very few studies. Our study found that mortality risk differed significantly by cohort age and sex. It might be that the risk is also higher for other subgroups, such as people living with HIV, 137 a possibility future research should investigate.
The number of available studies in some stratified analyses was small, so there may be limited power to control for potential study level confounders. However, the required number of estimates per variable for linear regression can be much smaller than in logistic regression, and a minimum of at least 2 estimates per variable is recommended for linear regression analysis, 138 suggesting the sample sizes were adequate in all models presented. It has been demonstrated that a pattern of binge (ie, heavy episodic) drinking removes the appearance of reduced health risks even when mean daily volume is low. 139 Too few studies adequately controlled for this variable to investigate its association with different outcomes across studies. Additionally, our findings only apply to the net effect of alcohol at different doses on all-cause mortality, and different risk associations likely apply for specific disease categories. The biases identified here likely apply to estimates of risk for alcohol and all diseases. It is likely that correcting for these biases will raise risk estimates for many types of outcome compared with most existing estimates.
This updated meta-analysis did not find significantly reduced risk of all-cause mortality associated with low-volume alcohol consumption after adjusting for potential confounding effects of influential study characteristics. Future longitudinal studies in this field should attempt to minimize lifetime selection biases by not including former and occasional drinkers in the reference group, and by using younger cohorts (ie, age distributions that are more representative of drinkers in the general population) at baseline.
Accepted for Publication: February 17, 2023.
Published: March 31, 2023. doi:10.1001/jamanetworkopen.2023.6185
Correction: This article was corrected on May 9, 2023, to fix errors in the Figure and Supplement.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Zhao J et al. JAMA Network Open .
Corresponding Author: Jinhui Zhao, PhD, Canadian Institute for Substance Use Research, University of Victoria, PO Box 1700 STN CSC, Victoria, BC V8Y 2E4, Canada ( [email protected] ).
Author Contributions: Drs Zhao and Stockwell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zhao, Stockwell, Naimi, Churchill, Sherk.
Acquisition, analysis, or interpretation of data: Zhao, Stockwell, Naimi, Clay.
Drafting of the manuscript: Zhao, Stockwell, Clay.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Zhao, Churchill.
Obtained funding: Zhao, Stockwell, Sherk.
Administrative, technical, or material support: Zhao, Stockwell, Naimi.
Supervision: Zhao, Stockwell, Naimi.
Conflict of Interest Disclosures: Dr Stockwell reported receiving personal fees from Ontario Public Servants Employees Union for expert witness testimony and personal fees from Alko outside the submitted work. Dr Sherk reported receiving grants from Canadian Centre on Substance Use and Addiction (CCSA) during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was partly funded by the CCSA as a subcontract for a Health Canada grant to develop guidance for Canadians on alcohol and health.
Role of the Funder/Sponsor: Health Canada had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. CCSA staff conducted a preliminary search to identify potentially relevant articles but did not participate in decisions about inclusion/exclusion of studies, coding, analysis, interpretation of results or approving the final manuscript.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We gratefully acknowledge contributions by Christine Levesque, PhD (CCSA), and Nitika Sanger, PhD (CCSA), who conducted a preliminary literature search for potentially relevant articles. We also acknowledge the leadership of Drs Catherine Paradis, PhD (CCSA), and Peter Butt, MD (University of Saskatchewan), who cochaired the process of developing Canada’s new guidance on alcohol and health, a larger project which contributed some funds for the work undertaken for this study. We are grateful to Fariha Alam, MPH (Canadian Institute for Substance Use and Research), for her help coding the studies used in this study. None of them received any compensation beyond their normal salaries for this work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Alcohol Consumption Patterns: A Systematic Review of Demographic and Sociocultural Influencing Factors
Abd alghani khamis, siti zuliana salleh, mohd sayuti ab karim, noor ashikin mohd rom, shamini janasekaran, rusdi bin abd rashid.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2022 May 12; Accepted 2022 Jun 28; Collection date 2022 Jul.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Background: Excessive alcohol consumption has negative effects not only on the drinkers’ health but also on others around them. Previous studies suggest that excessive alcohol consumption can be related to a combination of factors such as age, family background, religiosity, etc. Investigating and clarifying these roots of alcohol consumption is crucial so that the right type of interventions can be designed in a specific and targeted manner. Objectives: This work was conducted as a systematic review to reveal the factors associated with alcohol consumption and to heighten the understanding of the differences among various communities and segments of the population regarding their usage of alcohol. Data sources: A systematic search of Web of Science, PubMed, ScienceDirect, and Google Scholar was performed. Results: Forty-five studies were included in the review after excluding irrelevant records and duplicates. Conclusions: Alcohol consumption patterns can be associated with several factors related to communities and individuals, and our review revealed demographic factors, including age and proximity to alcohol outlets, as well as social factors, including family background, socioeconomic background, and religious influence. These findings can be used to establish a guideline for further studies in understanding alcohol consumption patterns among individuals according to their personal characteristics and sociocultural backgrounds.
Keywords: alcohol consumption patterns, physical and mental health, demographics, social factors
1. Introduction
Alcohol existence and consumption can be traced as far back as 7000 BC [ 1 ]. A number of medical studies have reported the positive effects of moderate alcohol consumption. For instance, it can prevent certain diseases and medical conditions, such as a heart attack [ 2 ]. It has also been observed that individuals can use alcohol as an aiding tool to control social conditions. For instance, Hajek et al. [ 3 ] found an association between decreased loneliness, higher life satisfaction, and less perceived stress with those who reported occasional and daily drinking. However, alcohol also possesses both toxic and intoxicating properties. Alcohol is a toxic substance that is foreign to the body (not produced by the body), and it can lead to serious poisonous effects, especially when taken in high concentrations [ 4 ]. In general, the harmfulness of alcohol consumption can be related to the total volume of irregular heavy drinking [ 5 , 6 ]. Furthermore, people who drink frequently in licensed establishments are more likely to be harmed by other’s drinking [ 7 ].
Awareness about the negative effects of alcohol consumption on physical and mental health has increased in recent decades. The main reason for this is that alcohol not only harms individual drinkers but also the wellbeing of their families and communities. In general, excessive alcohol consumption accounts for 5.1% of global diseases and injuries [ 8 ]. According to an investigation based on gender, harmful drinking accounts for 7.1% and 2.2% of global diseases for men and women, respectively [ 9 ]. Furthermore, alcohol consumption is also responsible for 10% of all deaths among people aged 15–49 [ 10 ]. Besides that, alcohol is the leading cause of premature death and disability of newborn babies [ 11 ]. Alcohol consumption also has other negative effects on individual performance, such as slower responses, particularly as a result of an alcohol hangover [ 12 ]. This was also observed by Aas et al. [ 13 ], who reported that employees’ consumption of alcohol was related to their performance both at the workplace and outside.
Furthermore, alcohol consumption can cause mortality in offspring due to suicide or violence. Landberg et al. [ 14 ] reported that the threat of violent death has been increasing among boys whose fathers are frequent consumers, and the threat of suicide increases in the highest category of fathers’ consumption. Heavy alcohol consumption and extreme weekly binge drinking have a larger effect on cognitive decline in an adult’s life [ 15 ]. Alcohol consumption adversely affects consumers’ driving performance, as it also has a degradation effect on vision [ 16 ]. Additionally, socioeconomic inequalities could result in inequal alcohol-related harm, despite similar consumed quantities [ 17 , 18 ]. Although a number of interventionist approaches have been implemented by governments to lower the rate of alcohol consumption, life-threatening situations due to alcohol abuse still prevail [ 19 ].
To address the problems associated with alcohol abuse, it is necessary to investigate and understand the reasons for alcohol consumption among different communities and segments of the population. Survey investigations regarding alcohol consumption have been widely reported [ 20 , 21 , 22 ]. According to the literature, alcohol consumption patterns can be related to life events [ 23 ], as well as demographic and sociocultural factors [ 24 ]. For instance, it can be related to age [ 25 , 26 , 27 , 28 ], socioeconomic background [ 29 ], and family background [ 30 ].
This review paper examines the different approaches to study alcohol consumption among different communities and segments of the population. It also discusses the relationship between risky alcohol consumption and various personal demographics and sociocultural factors among individuals and communities. A qualitative hypothesis was formed to drive the search of this review and was broken down into several questions as follows:
Q1: How can different factors among individuals and communities such as age, family background, or socioeconomic conditions be used as predictors of alcohol consumption patterns?
Q2: Does the extant literature include an adequate investigation of the abovementioned associations?
Q3: What are the strengths and weaknesses of earlier studies that can serve as a guide for future related research?
2. Methodology
Our research was conducted mainly on the Web of Science, PubMed, ScienceDirect, and Google Scholar search engines. Other sources of data were also used, including the World Health Organization’s formal website and manual tracking of cited records. The study focused on acquiring published articles related to alcohol use and related influencing factors in general. The query used to obtain the records used in this review was a combination of alcohol-related keywords and influencing factor keywords, as shown in Table 1 . These combinations were (“Alcohol misuse” OR “Alcohol consumption” OR “Alcohol consumption patterns”) AND (“Socioeconomics” OR “Age” OR “Family” OR “Influencing factors” OR “Proximity to alcohol outlets” OR “Alcohol outlets” OR “Religiosity influence” OR “Religion”).
Quality assessment of the observational studies.
The eligibility for study inclusion was limited to studies that were published in the English language, scored 50% and above using the Quality Assessment Tools developed by the National Heart, Lung, and Blood Institute, and published within the past six years (2017–2022). We used EndNote X8 during the entire screening process and Mendeley as a citing manager. Screening the records focused on collecting information about the causes of more alcohol consumption. We screened the records for the inclusion criteria where only records concerning the first research question specified earlier were chosen. The other two research questions were answered later based on the results of the quality assessment.
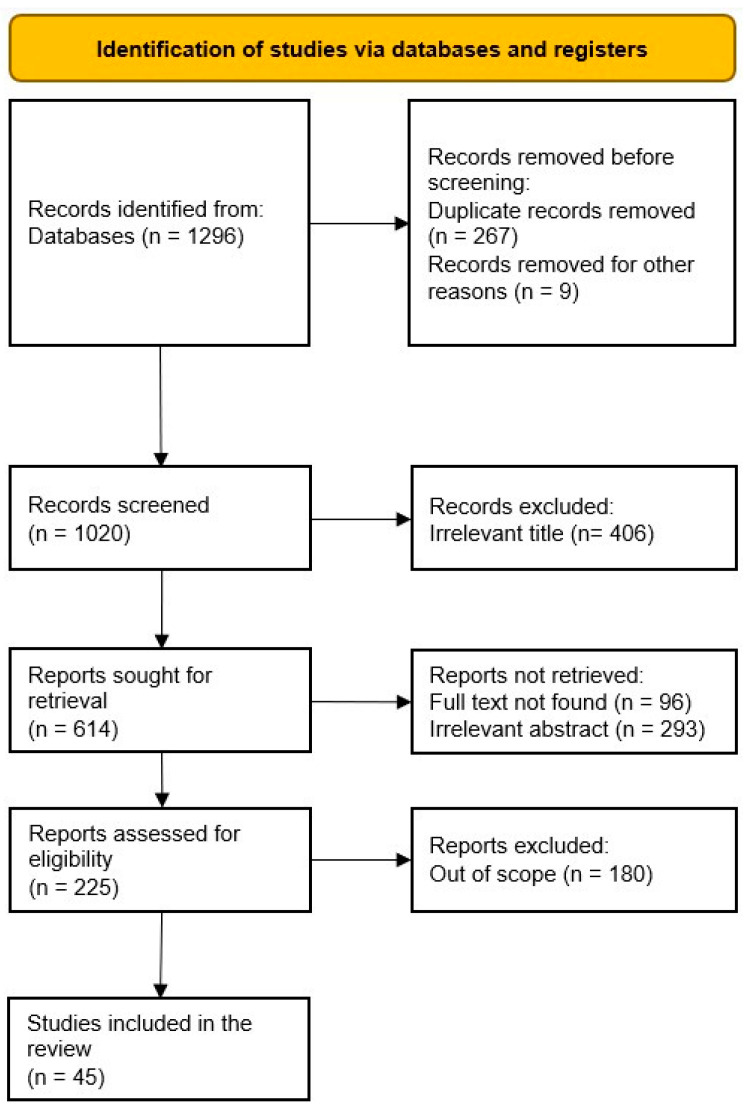
According to the PRISMA guidelines, a systematic review is a survey that utilizes express, orderly techniques to group and integrate discoveries of studies that address an obviously figured-out question [ 31 ]. We followed the PRISMA throughout the screening process and the designing of the methodology. Figure 1 shows the abstract of the PRISMA flow of the review. We began by designing the research, then collecting all identified records and extracting the data. At a later stage, an assessment of the quality of the studies was performed, whereby the National Heart, Lung, and Blood Institute’s Study Quality Assessment Tool was used to assess the quality of the studies involved in the qualitative synthesis [ 32 ]. A. Khamis and S.Z. Salleh were involved in data extraction and collection for all identified records, and A. Khamis worked on the assessment of the study quality independently while S.Z. Salleh reviewed the assessment. No meta-analysis was performed due to the variety of measures and outcomes.
PRISMA flow diagram.
The systematically identified papers were assessed using The National Heart, Lung, and Blood Institute’s (NHLBI, July 2021) quality assessment tool to rate the quality of the evidence for the observational studies. The NHLBI tool assessed the quality of publication using several criteria, as listed below:
1 = Research questions clearly stated;
2 = Study population clearly defined;
3 = Participation rate ≥50%;
4 = Subjects from the same population and inclusion/exclusion criteria specified;
5 = Sample size justification included;
6 = Exposure measured before outcome;
7 = Sufficient timeframe to see an effect;
8 = Different levels of exposure included;
9 = Clearly defined exposure measures;
10 = Exposures measured more than once;
11 = Clearly defined outcome measures;
12 = Outcome assessors blinded to exposure status;
13 = Loss to follow-up ≤ 20%;
14 = Confounders measured and adjusted for.
This review was conducted to explore the association between demographic and/or social characteristics and alcohol usage; consequently, publications that documented the effects of alcohol were excluded from the quality rating process. To avoid any possible bias in the quality ranking process, the studies were ranked based on the given criteria before sorting them into categories. The findings of each study were later sorted into categories to reveal the different associations. In case of finding conflicts, each kind of association was sorted individually within the category itself to be compared and thoroughly discussed later. If a study had multiple findings that can be sorted into different categories, the ranking of the study will be used in every relevant category, and the summary of the findings will be divided into different categories.
The ranking for criteria 1 and 2 was done straightforwardly just by reading through the paper and trying to identify the information regarding the research questions and the population, while, for criteria 3, the assessment was based on a deeper search. For instance, if the study was sampling data from a bigger study or survey, we looked for the source of these data and identified the original response rate of the initial sample and then ranked them accordingly. Additionally, the response rate for studies that were multi-nationals or sampling data from multiple sources was assessed by calculating the average response rate across all sources. A point for criteria 4 was given if the study specified the inclusion/exclusion criteria of the population or if the information about the sample was clear enough for the study to be done again. Studies that did not justify their sample size or provide a statistical power calculation were not given criteria 5. If the study included information about the exposure measurements before the outcome assessment and if the study was cross-sectional yet the exposure took time previously, such as when sampling from a religious affiliating university to examine the associations between religiosity and consumption, criteria 6 was given due to the fact that the exposure to religion was present before the study. Some other studies collected data from participants at a specific point of time without analyzing the exposure time frame; those studies did not get points for criteria 7. However, we looked into the studies more thoroughly, and in cases where the exposure took enough time to have an influence on the participant’s life before the study took place, then we gave that a point for criteria 7; for example, under religious influence, if the participants had enough time to be involved in the religion, were the proper age, or living with parents following that religion, then they got a point for criteria 7 under the assumption that the sample had enough time to be influenced by this exposure. Points for criteria 8, 9, 10, and 11 were assessed by looking for straight answers within the study texts. A point for criteria 12 was not given for studies that did not include information about the blinded assessors, as we assumed they were blinded for studies analyzing secondary data (e.g., government surveys); however, when both outcomes and exposures were assessed by the same survey and timeframe, a point for criteria 12 was not given. For criteria 13, the follow-up retention rate was retrieved by either looking through the original source of data for each study or from within the study itself if it was reported. Finally, criteria 14 was assessed by looking at the statistical analysis and the discussion of the results.
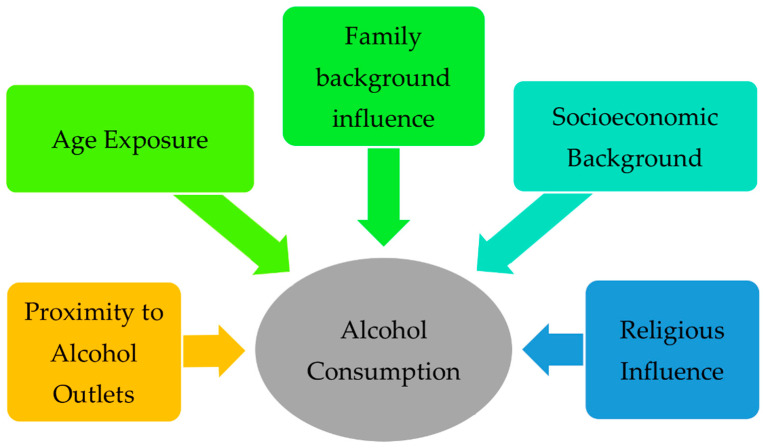
The influencing factors were extracted from the studies only if they were viewed as an independent variable and had statistical significance. The influencing factors were categorized into five categories: proximity to alcohol outlets (physical exposure), age exposure, family influence, and socioeconomic and religious influence. The proximity to alcohol outlets associated with alcohol consumption combined both on-premises and off-premises outlets and investigated the drinking patterns associated with the outlet density in general. The age exposure included the influence of early access to alcohol, the influence of aging on the consumption patterns, and the influence of the age of their first drink. Family influence included the effect of parenting authority, the effect of family instability, and familial exposure. In the socioeconomic exposure category, the direct influences of socioeconomic status at both the individual and collective levels were included. Finally, religious influences were just discussed in a general matter to reveal whether religiosity has an influence on the drinking profile without any comparisons between different religions.
As indicated in the PRISMA diagram ( Figure 1 ), we were able to collect 1296 records using the query mentioned above. The total number of reports and studies included in this review was 45 after removing the duplicates (using the EndNote automated tool), irrelevant records (by screening titles and abstracts), and records written in other languages but English (by screening the full texts). Most of the studies reported multiple findings; however, this did not affect our data extraction, since the study focused solely on the association between demographic/social factors and alcohol consumption patterns and hence extracted the related findings and included them in the appropriate category individually.
The overall percentage of the quality assessment criteria met for all the findings (n = 51) was 67.85%, with an average met criteria of 9.5 ( Table 1 ). Criteria number 1 (research question clearly stated), number 2 (study population clearly defined), number 4 (clear population and inclusion/exclusion criteria), number 8 (different levels of exposure included), and number 11 (defined outcome measures) were satisfied by all the findings, while the most unsatisfied criteria were number 5 (sample size justification included), with only 10 studies satisfying it, criteria number 10 (exposures measured more than once), with 12 studies satisfying it, and criteria number 13 (follow-up with a loss less than 20%), with 13 studies satisfying it. These findings provide lessons for establishing better research designs in the future to avoid the risk of bias.
For the association with physical exposure to alcohol outlets, 12 studies were identified. Eleven studies reported a positive association with a higher density of alcohol outlets and an average quality of evidence score of 10.1 (72.14%), mostly lacking sample size justifications and follow ups, while the other study that did not report a positive association only reported neutral effects, concluding that an increased density of outlets did not show an increment in either heavy drinking or AUDIT scores. That study scored 9 points (64.29%) for the quality criteria.
For the age exposure category, 10 studies were identified. These studies were divided into three different types of associations: early access to alcohol, aging, and age of onset, with four studies identified for early access and three studies for aging and the age of onset. The highest overall score for the quality of evidence rank was for the early access association, satisfying 78.57% of the quality criteria on average, while aging scored the lowest average of 62.42%, and the age of onset scored 68.57% on average.
The family background influence was also backed up with 10 studies divided into three categories: parenting authority, family instability, and family exposure. For parenting authority, two studies were identified related to permissive parenting authority with conflicting findings; one was positively associated, with a 57.14% quality rank, compared to the other negatively associated with a 50% quality rank. Similarly, two studies were sorted into the family instability category, both studies had a positive correlation with alcohol consumption, with an average 64.29% quality rank. Finally, the remaining six studies were sorted into family exposure association; five were positively associated with predicting future alcohol consumption, with an average quality score of 71.42%, and one was neutral, with a quality of evidence score of 78.57%. A more thorough breakdown of these findings will be done in the Discussion section.
In the socioeconomic exposure category, the findings were either on a collective level (overall socioeconomic condition of a nation or a group) or at an individual level (personal monthly income or individual status of wealth). Both the collective level and individual level were used to show the associations; however, there was some conflict in the findings. We found six studies to be positively associated with alcohol consumption (65.71% average quality rank) and two negatively associated (67.85% average quality rank).
Finally, 11 studies were sorted into religious influence; all of them reported a negative association with alcohol consumption, with an average quality rank of 63.57%, most lacking criteria number 10 (exposure measured more than once) and all lacking criteria number 5 (sample size justification).
4. Discussion
As mentioned earlier, the roots of alcohol consumption in the community need to be investigated to reduce and treat excessive or irregular heavy drinking. Based on a systematic literature review, we can extend our current understanding of the factors associated with alcohol consumption. These factors can be categorized as either demographic (proximity and age) or social (family influence, socioeconomic background, and religious influence), as illustrated in Figure 2 . The studies in the literature had a wide variety of outcomes, including frequency, volume per day, volume per week, binge drinking, and alcohol-related problems. In the coming sections, the findings of these studies will be discussed and explained thoroughly.
Factors associated with alcohol consumption patterns.
4.1. Proximity to Alcohol Outlets
One of the main factors influencing alcohol consumption is the proximity to alcohol outlets. The more outlets are made available, the easier the accessibility is to alcohol. For instance, a study by Toornstra et al. [ 36 ] reported that easy availability, low pricing, and peer pressure contribute to more alcohol consumption among adults, young adults, and adolescents in general. Among adolescents, alcohol consumption by peer access was reported to be higher with exposure to alcohol outlets [ 33 ]. In agreement with Morrison and colleagues, Martins et al. [ 37 ], in a convenience sampling method, discovered that binge drinking among students often occurs as a result of the density of alcohol outlets near the school; however, the convenience sample was not sufficient enough to be representative [ 75 ]. Correspondingly, a cross-sectional study with a randomly drawn sample from Curitiba’s public schools conducted by Cardoza et al. [ 41 ] showed an agreement with the mentioned association between adolescents’ consumption and the alcohol outlet density, where it reported that a higher density of alcohol outlets is positively associated with more consumption, and adolescents in schools located further than 250 m away from alcohol outlets were had lower consumption of alcohol.
A similar relationship between women and alcohol outlets with alcohol consumption was also reported. Lamb et al. [ 35 ], in a cross-sectional study sampling 995 women, reported that increasing the number of alcohol outlets within as little as a 3-kilometre radius can be linked to higher levels of the harmful consumption of alcohol among women. Concordantly, Seid et al. [ 34 ] stated that more reports regarding harmful effects, such as in marriage, relationships, or finance, have been observed in women who live nearer to alcohol outlets.
Similar associations between a higher density of alcohol outlets/liquor licenses and more alcohol consumption can be found in different countries. For instance, the density of alcohol outlets moderated the heritability of alcohol problems in a study sampling from Germany, the United Kingdom, France, and Ireland [ 23 ]; the alcohol consumption and mean daily intake of alcohol increased with the liquor license increments in two study samples from Western Australia [ 38 , 39 ]; the number of drinks and high consumption in general were positively associated with a high outlet density in Philadelphia, Pennsylvania [ 40 ]; and binge drinking was reported to be increasing on a daily, weekly, and yearly basis with the higher density of alcohol outlets in Mexico [ 76 ].
One recent study conducted by Mair et al. [ 42 ] in Alameda County, California, found a neutral association between the density of alcohol outlets and increased heavy drinking. However, this finding was limited to off-premise outlets and heavy drinking only. Additionally, this study lacked information about response rate, which is an important indicator of the representativity of the outcome.
Looking at the evidence, a higher density of alcohol outlets may be a contributing factor to the more frequent consumption of alcohol, which can be explained by easier sourcing of the substance. Although the outcomes and outlet types that were examined by the studies were various, this factor holds a correlation with alcohol consumption globally; however, that relation may differ between regions. For instance, in Europe, the association with increased outlets moderates the heritability of alcohol outlets; in Australia, it was related to the mean daily intake; and in North America, it was found to be related to high consumption. The unique effect of off-premise and on-premise outlets was not revealed clearly, which may require an additional investigation specified to each type of outlet.
4.2. Age Exposure
Another factor related to alcohol consumption is age exposure. After being allowed to consume alcohol for the first time, adolescents are more likely to progress from drinking one drink to five or more at once [ 44 ]. Not only does early access predict consumption profiles but so does the trajectory; a study by Plenty et al. [ 46 ] showed that it is twice as likely to get high AUDIT scores and social harm and triple the odds of heavy episodic drinking for a steep escalator trajectory of young age compared to those with a slow increment of alcohol use.
Soundararajan et al. [ 45 ] reported an association between early access to alcohol and the frequency of alcohol consumption in a study sampling 99 participants from addiction wards in India, using questionnaire data; more frequent consumption and a higher frequency of heavy consumption were positively associated. This association was also observed among students from two different nations in a study sampling 1833 13-years-old students from Washington, USA and Victoria, Australia, conducted by Kim et al. [ 43 ], who showed that the early use of alcohol predicts frequent drinking and alcohol problems later in life.
The age at which the first alcoholic drink was consumed can be important to account for, since it may have effects on the awareness and knowledge development regarding responsible alcohol consumption. Aguilar et al. [ 50 ] observed that higher rates of drinking were among those with early onset regardless of sex. Another study that accounted for possible confounders and included parent–child dyads found that early age of onset can be linked with both frequent and infrequent binging, and initiation as early as 13 years-old or less is even more at risk of increasing frequent binging and the total volume of drinks, while later initiation (16 to 17 years old) seemed to be associated with a reduced risk of infrequent binging [ 52 ]. Additionally, Islam [ 51 ] analyzed the survey data from multiple years and found an association between age of onset and awareness of low-risk drinking and drinks counting, where a higher awareness was observed among those initiated later, especially during adolescence. These findings can be helpful, especially for parenting, since parenting authority itself has been found to have an effect, as will be discussed in the next subsection.
As discussed above, alcohol consumption in heavy quantities might be associated with younger ages. Older ages may have less risky consumption or less consumption at all, depending on the norms and culture in the country of residence. For instance, a study conducted in Malaysia discovered that alcohol consumption odds can decrease with aging up to 0.016 times with every additional year of age [ 49 ], while, in other countries, it may be different. For instance, a study sampling from Australia, England, Scotland, New Zealand, St Kitts and Nevis, Thailand, South Africa, Mongolia, and Vietnam discovered that the drinking frequency in general increases with the age increment; however, the consumption quantity is not likely to be large, and this association was more consistent among higher-income countries [ 48 ]. In another study sampling citizens aged more than 50 years from 12 different European countries, both males and females showed a substantial relationship between age and alcohol consumption, with consumption dropping as they got older [ 47 ].
The revealed association with age may draw a timeline for the relationship between the age and consumption, where the early years of life are crucial to build an appropriate awareness about substance use, and the consumption patterns during those years may have an effect on the later stages; a younger age of exposure was related to riskier patterns and consequences, and older ages, even though they were observed to have higher consumption frequencies, showed better awareness, since the quantities were not observed to be high.
The association with older age may differ between regions. Although the study conducted in Malaysia by Kang Cheah & Rasiah [ 49 ] revealed that the consumption odds reduced with aging, the study reported by Chaiyasong et al. [ 48 ] was done on several countries, including countries from the same region as Malaysia, which revealed the opposite association, where the frequency increased with aging. It is worth noting that the study done by Kang Cheah & Rasiah [ 49 ] used the past 30 days of consumption and consumption during the data collection as a measurement of the outcome and included all types of consumption, while the latter study used frequency, the typical occasion quantity, and volume to determine the outcome. All things considered, aging may be associated with more frequency of consumption; however, that increased frequency does not seem to be alarming, since the quantities are not likely to be large. Nevertheless, frequency and quantity both play a sensitive role at young ages, and efforts should be made for preventive measures for this population.
4.3. Family Background Influence
Indeed, family has an influence in the formation of one’s personality and lifestyle and that includes the stability of the family, parenting authority, and the lifestyle of the family. In our review, we collected studies relating these factors to alcohol consumption.
At a young age, the parenting authority plays an important role, and as discussed earlier, the age of the first drink and the early access of alcohol play important roles in developing a proper awareness and drinking patterns. According to the literature, permissive parenting authority can act differently. For example, Mathialagan et al. [ 53 ] used AUDIT scores and surveys to collect data from 150 college students; the findings indicated that increased authority did not impact the consumption patterns significantly, while permissive authority was found to decrease the consumption patterns. Another study conducted by Dickens et al. [ 54 ] used survey data from 23,163 rural adolescents and found that increased parental permissiveness increased the likelihood of alcohol use the previous month, which was opposite to the earlier study. However, it is worth mentioning that the earlier study surveyed college students from SEGi College, Malaysia, which is a private university with diverse nationalities and different backgrounds, while the latest one collected survey data from middle and high school students from non-metropolitan counties in the U.S. Both studies did not include sample size justifications, and they did not measure and account for confounders such as early access or age of onset. However, the latter study used data from a larger data collection effort, which may indicate blinded assessors, which gives more strength to the latter study. Additionally, the population of the studies were different, college students from a private university vs. rural adolescents, so more studies are needed to clarify this association, and accounting for the population should take place.
The other factor in family background is family instability. According to the literature, being widowed or divorced is linked to more problematic alcohol use [ 55 ]. Nonintact and complex family structures may be linked to alcohol misuse among adolescents [ 56 ].
Families’ alcohol dependence can also contribute. For instance, it was reported that the amount of alcohol consumed by parents and siblings has a significant impact on the amount of alcohol consumed by other siblings [ 59 ]. Additionally, having a family history of alcohol use for those with high Barratt Impulsiveness Scale scores (a questionnaire designed to assess the personality/behavioral construct of impulsiveness) was associated with severe alcohol-related consequences [ 57 ]. Living with parents with an alcohol use disorder may results in the earlier consumption of alcohol, and it was reported that male patients who lived with both parents with an alcohol use disorder were younger than the female patients and patients with parents without an alcohol use disorder when they first consumed alcohol [ 58 ]; however, this study had significantly more male participants than females, which could alternate the results when comparing males and females.
Mothers might have more impact than fathers, as one study reported that drinking increased in women who said their mother was a heavy or problem drinker and who thought they were like their mother, while the fathers’ results did not follow the same pattern [ 60 ]. In another study done by Tschorn et al. [ 23 ], early hazardous drinking (at the ages of 14–16) was linked to the mother’s exposure to alcohol during pregnancy.
In a study utilizing self-answered questionnaire sampling from grade 7 classes from private schools in Australia, neither of the above associations was discovered, only proving that accessing alcohol through a parental supply only allowed for consumption during the adolescent years without any prediction of future prevention or protection from frequent consumption, as an increase in alcohol consumption kept taking place throughout the adolesce years, regardless of the parental supply [ 61 ]. Although this study had a considerable high quality of evidence (78.57%), its findings may not be generalized for later ages, since the follow-up continued until grade 10, and nothing was reported for the later years.
The studies concerning parental authority did not yield a consistent association. Moreover, the study conducted by Clare et al. [ 61 ], despite its limited investigation timeframe, showed that an increase in consumption kept taking place regardless of the parental supply (which may indicate permissive parenting). However, the age at which parental authority is present the most showed an importance in the formation of the awareness regarding responsible alcohol consumption, as discussed in the previous subsection. Other familial factors may present an additional contribution. For instance, the consumption by parents, siblings, and historical familial exposure did show an association, with agreements across all identified studies.
All things considered; parental permissiveness combined with the familial background may have a unique impact, since parental authority alone would not be enough to raise the awareness of children when they may imitate a familial model that was present throughout their lives.
4.4. Socioeconomic Exposure
Socioeconomic exposure can be extended to the level of education, employment, type of profession, and monthly income, as all these factors may expose individuals to certain alcohol consumption patterns, including risky consumption. In terms of alcohol consumption in general, it was reported that men with a higher socioeconomic status were more likely to drink alcohol and smoke [ 62 ], professionals and mangers had more drinking occasions than semi-skilled and unskilled manual workers [ 29 ], and higher chances of alcohol consumption could be found among students who worked [ 41 ].
A study conducted on Malaysian participants confirmed this association, where it was found that there were 0.004 times more odds of alcohol consumption with every MYR 100 increment to the monthly income [ 49 ]. In a later study, Mair et al. [ 42 ] demonstrated the same association between high income and the frequency of consumption, as well as another finding that residents of a high-income area consume more than residents of a low-income area, regardless of the income level. Additionally, a study sampling from multiple countries discovered a similar overall association between income level and frequency of consumption, whereas high-income countries showed more frequency of consumption than middle-income countries, with the exception of one middle-income country, which is South Africa [ 48 ].
Although the above-mentioned studies showed a positive association between high income and consumption, other studies reported a positive association with low income and alcohol consumption. For instance, Čihák [ 63 ] studied data from statistics departments in the Czech Republic and found that economic downturns resulted in higher alcohol consumption, which could be related to the increase in the unemployment rate. Additionally, Khan & Shaw [ 64 ] discovered a higher alcohol consumption among the ST class in India (which is socially excluded and in a lower wealth category).
The average rank of the quality of evidence for both associations was similar: 65.7% for the positive association and 67.8% for the negative; however, the number of studies was not the same, with triple the number of studies concluded with a positive association with a high income. Additionally, the Čihák [ 63 ] and Khan & Shaw [ 64 ] studies both could not be generalized, since the first one used data about alcohol-related liver cirrhosis to indicate the overall increased consumption of alcohol, which risked insufficient representation of the population, and the latter had a confounder, which was the social exclusion of the specific group, which may have shifted the findings of the study towards the group.
In general, an increased income allows individuals to afford more products, including alcoholic products, than those with limited incomes. Additionally, the time availability may increase among those with a higher socioeconomic status, especially when the professions are different. For example, professionals and managers may need to work less hours than unskilled workers, who might require more working hours to generate an income. However, students who worked were observed to be exposed to alcohol consumption; this may not be linked to more time availability but to a higher ability to afford alcoholic products. Despite that, affording the product may not be the only contributing factor, as being socially excluded or going through an economic downturn at a large scale could result in exposure, regardless of the individual’s wealth level. Nevertheless, socioeconomics can be associated with an alcohol consumption pattern; however, the degree to which this association could be present may vary according to the overall current social and economic situations of a community.
4.5. Religious Influence
Religion influences individuals’ perceptions and attitudes towards alcohol and, consequently, their approach to alcohol use. Additionally, the strength of religious involvement is a big factor in protection against alcohol consumption [ 73 ]. Notably, religiosity has a negative relationship with excessive alcohol intake, whereas undergraduates who reported alcohol-related problems were found to be less religious [ 69 ]. Higher religiosity results in less odds of engaging in binge drinking [ 70 ]. Higher religiosity is also connected with less alcohol consumption frequencies for both genders [ 68 ]. Similarly, another study indicated that religiosity is linked to a reduction in alcohol usage in general, and nonbelievers consume alcohol and misuse it the most, relative to Catholics and Muslims [ 65 ]. Religious activities have been investigated as well. For instance, religious chanting/singing and reading the sacred texts are associated with lower alcohol consumption in general [ 72 ]; however, it was alco reported that frequent prayer can aid alcohol consumption reduction for moderate drinkers but not heavy drinkers [ 66 ].
One’s feelings toward religion and its importance in life may moderate the effects of religiosity. For instance, those who believed their religious heritage is prescriptive drank less alcohol and had stronger religious characteristics [ 72 ]. On the other hand, students who reported that religion played a minor role in their lives were more likely to have recently consumed alcohol [ 67 ]. Nevertheless, the presence of a religious affiliation is linked to less frequent alcohol usage [ 73 ], and religious commitment is associated with a reduced likelihood of substance misuse [ 74 ].
Religiosity may impact communities differently. For instance, in a study analyzing survey data from adolescents from rural communities, it was reported that religiosity was associated with less recent alcohol consumption but with a bigger influence on White teenagers than African Americans [ 54 ], while another study utilized data from a nationally representative sample of individuals aged 17–31 years from the U.S. and reported the same link between increased religiosity and a lower risk of any substance use; however, this link may weaken with age [ 74 ]. Basically, the effects of religious involvement can vary according to factors such as whether its involvement was during childhood or adulthood, and that too can vary according to other factors, such as ethnicity, according to Agrawal et al. [ 71 ], who reported that adulthood involvement with religion showed decreased alcohol involvement for both Black and White ethnic groups, unlike childhood involvement, which demonstrated an association only for the White group.
In general, religious involvement may be linked to more responsible alcohol consumption patterns. Although it was observed that the strength of this association may differ between populations, the exposure itself may moderate this outcome. Since more involvement with religion will yield more religious activities, such as chanting/signing and that was linked to lower alcohol consumption, this can be explained as, the larger the role religion plays in one’s life, the more effect that religion will potentially have on consumption patterns.
5. Conclusions
In this paper, based on a systematic review of the extant literature, we conclude that alcohol consumption can be attributed to a range of demographic and social factors, namely access to alcohol outlets, age exposure, familial background, socioeconomic background, and religious influence. Easier access to the substance can be associated with the more frequent consumption of alcohol among adults, young adults, and adolescents in general, and it may be associated with higher levels of harmful consumption, as well as more harmful effects in life events for women. Adolescents exposed to alcohol at a young age, regardless of gender, drank more, but their drinking habits changed as they grew older. Family background influences alcohol dependence, including family stability, parental authority over early access to alcohol, and family lifestyle, which influences alcohol dependence, notably mother drinking tolerance and habit of consuming alcohol throughout pregnancy. The level of education, employment, type of profession, and monthly income can have an impact on alcohol consumption. There is a link between high religiosity and fewer alcohol-related problems, less binge drinking, and less alcohol consumption frequency for any gender. Identifying the factors associated with alcohol misuse is crucial so that the right policy and community-level prevention interventions can be provided to populations with potential substance use disorders and those affected by their negative behaviors. Understanding these factors can lead to better guidelines for alcohol use and can aid in better designs for prevention interventions.
6. Limitations and Outlook
The scope of this review was limited to the factors identified through the search terms, leaving behind other factors that might have had evidence of the same caliber or even greater quality. Future reviews might concentrate on addressing additional possible factors such as the effects of peer pressure and marital status. Due to the data collection being limited to the last six years (2017–2022), some significant findings may have been missed.
Additionally, the studies identified from the search were observational studies, which can be understood, as conducting a controlled trial to measure the effect of exposure would be a huge challenge and risk exposing the research subjects to alcohol misuse.
One other limitation of our review was the influence of proximity to alcohol outlets, where we reviewed the influence of alcohol outlet density in general. However, we did not investigate the difference between on-premise and off-premise outlets, which may need further understanding. The findings in this review suggest that a wide range of factors can contribute to alcohol consumption profiles, awareness, and behaviors.
Author Contributions
Conceptualization, M.S.A.K., A.A.K. and S.Z.S.; methodology, M.S.A.K. and S.J.; validation, S.J. and A.I.; formal analysis, A.A.K. and S.Z.S.; data curation, A.A.K. and S.Z.S.; writing—original draft preparation, A.A.K. and S.Z.S.; writing—review and editing, N.A.M.R., A.I. and R.B.A.R.; visualization, S.Z.S.; supervision, S.J. and R.B.A.R.; project administration, M.S.A.K.; funding acquisition, M.S.A.K. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Universiti Malaya Impact-Oriented Interdisciplinary Research Grant Programme (grant number IIRG005C-2020SAH).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. McGovern P.E., Zhang J., Tang J., Zhang Z., Hall G.R., Moreau R.A., Nunez A., Butrym E.D., Richards M.P., Wang C.-S., et al. Fermented Beverages of Pre- And Proto-Historic China. Proc. Natl. Acad. Sci. USA. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Chevli P.A., Aladin A.I., Kanaya A.M., Kandula N.R., Malaver D., Herrington D.M. Alcohol consumption and subclinical atherosclerosis among South Asians: Findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Nutr. Metab. Cardiovasc. Dis. 2020;30:123–131. doi: 10.1016/j.numecd.2019.07.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Hajek A., Bock J.-O., Weyerer S., König H.-H. Correlates of alcohol consumption among Germans in the second half of life. Results of a population-based observational study. BMC Geriatr. 2017;17:207. doi: 10.1186/s12877-017-0592-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Hendriks H.F. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020;11:1–21. doi: 10.1146/annurev-food-032519-051827. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Maisto S.A., Galizio M., Connors G.J. “Alcohol,” in Drug Use and Abuse. 8th ed. Cengage Learning; Boston, MA, USA: 2018. [ Google Scholar ]
- 6. Poli A., Visioli F. Moderate alcohol use and health: An update a Consensus Document. BIO Web Conf. 2015;5:04001. doi: 10.1051/bioconf/20150504001. [ DOI ] [ Google Scholar ]
- 7. Moan I.S., Brunborg G.S. Alcohol’s Harm to Others: Does the Drinking Location Matter? Subst. Use Misuse. 2021;56:1421–1427. doi: 10.1080/10826084.2021.1928215. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. World Health Organization . Global Status Report on Alcohol and Health 2018. WHO; Geneva, Switzerland: 2018. [ Google Scholar ]
- 9. World Health Organization Alcohol: Overview. 2018. [(accessed on 30 April 2021)]. Available online: https://www.who.int/health-topics/alcohol#tab=tab_1 .
- 10. Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R.M., Tymeson H.D., Venkateswaran V., Tapp A.D., Forouzanfar M.H., Salama J.S., et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. World Health Organization . World Health Statistics 2020: Monitoring Health For. The Sdgs, Sustainable Development Goal. WHO; Geneva, Switzerland: 2020. [ Google Scholar ]
- 12. Alford C., Martinkova Z., Tiplady B., Reece R., Verster J.C. The Effects of Alcohol Hangover on Mood and Performance Assessed at Home. J. Clin. Med. 2020;9:1068. doi: 10.3390/jcm9041068. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Aas R.W., Haveraaen L., Sagvaag H., Thørrisen M.M. The influence of alcohol consumption on sickness presenteeism and impaired daily activities. The WIRUS screening study. PLoS ONE. 2017;12:e0186503. doi: 10.1371/journal.pone.0186503. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Landberg J., Danielsson A.-K., Falkstedt D., Hemmingsson T. Fathers’ Alcohol Consumption and Long-Term Risk for Mortality in Offspring. Alcohol Alcohol. 2018;53:753–759. doi: 10.1093/alcalc/agy058. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Grønkjær M., Flensborg-Madsen T., Osler M., Sørensen H.J., Becker U., Mortensen E.L. Adult-Life Alcohol Consumption and Age-Related Cognitive Decline from Early Adulthood to Late Midlife. Alcohol Alcohol. 2019;54:446–454. doi: 10.1093/alcalc/agz038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Casares-López M., Castro-Torres J.J., Martino F., Ortiz-Peregrina S., Ortiz C., Anera R.G. Contrast sensitivity and retinal straylight after alcohol consumption: Effects on driving performance. Sci. Rep. 2020;10:13599. doi: 10.1038/s41598-020-70645-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Boyd J., Bambra C., Purshouse R., Holmes J. Beyond Behaviour: How Health Inequality Theory Can Enhance Our Understanding of the ‘Alcohol-Harm Paradox’. Int. J. Environ. Res. Public Health. 2021;18:6025. doi: 10.3390/ijerph18116025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Borrell C., Palència L., Bosakova L., Gotsens M., Morrison J., Costa C., Dzurova D., Deboosere P., Lustigova M., Marí-Dell’Olmo M., et al. Socioeconomic Inequalities in Chronic Liver Diseases and Cirrhosis Mortality in European Urban Areas before and after the Onset of the 2008 Economic Recession. Int. J. Environ. Res. Public Health. 2021;18:8801. doi: 10.3390/ijerph18168801. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Park S.H., Kim D.J. Global and regional impacts of alcohol use on public health: Emphasis on alcohol policies. Clin. Mol. Hepatol. 2020;26:652–661. doi: 10.3350/cmh.2020.0160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Andreas J.B. Perceived harmfulness of various alcohol- and cannabis use modes: Secular trends, differences, and associations with actual substance use behaviors among Norwegian adolescents, 2007–2015. Drug Alcohol Depend. 2019;197:280–287. doi: 10.1016/j.drugalcdep.2019.02.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. National Institutes of Health . National Health and Morbidity Survey 2019 Non-Communicable Diseases, Healthcare Demand, and Health Literacy. Ministry of Health Malaysia; Putrajaya, Malaysia: 2019. [ DOI ] [ Google Scholar ]
- 22. Begun A., Clapp J.D. The Alcohol Misuse Grand Challenge Collective Reducing and preventing alcohol misuse and its consequences: A Grand Challenge for social work. Int. J. Alcohol Drug Res. 2016;5:73–83. doi: 10.7895/ijadr.v5i2.223. [ DOI ] [ Google Scholar ]
- 23. Tschorn M., IMAGEN Consortium. Lorenz R.C., O’Reilly P.F., Reichenberg A., Banaschewski T., Bokde A.L.W., Quinlan E.B., Desrivières S., Flor H., et al. Differential predictors for alcohol use in adolescents as a function of familial risk. Transl. Psychiatry. 2021;11:1–11. doi: 10.1038/s41398-021-01260-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Kumar J.V., Francis A. The Causes, Consequences. Types of Alcoholism and Role of Supervisor for Alcoholic Labourers in Workplace–An Overview. Res. J. Humanit. Soc. Sci. 2018;9:35. doi: 10.5958/2321-5828.2018.00007.4. [ DOI ] [ Google Scholar ]
- 25. Labots M., Cousijn J., Jolink L.A., Kenemans J.L., Vanderschuren L.J.M.J., Lesscher H.M.B. Age-Related Differences in Alcohol Intake and Control Over Alcohol Seeking in Rats. Front. Psychiatry. 2018;9:419. doi: 10.3389/fpsyt.2018.00419. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Cousijn J., Luijten M., Ewing S.W.F. Adolescent resilience to addiction: A social plasticity hypothesis. Lancet Child Adolesc. Health. 2018;2:69–78. doi: 10.1016/S2352-4642(17)30148-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Sohn A., Jang S. Do Drinking Norms, Motives, and Drinking Behaviors Differ by Age Group among Korean Women? Int. J. Environ. Res. Public Health. 2022;19:3345. doi: 10.3390/ijerph19063345. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Varlinskaya E.I., Kim E.U., Spear L.P. Chronic intermittent ethanol exposure during adolescence: Effects on stress-induced social alterations and social drinking in adulthood. Brain Res. 2017;1654:145–156. doi: 10.1016/j.brainres.2016.03.050. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Beard E., Brown J., West R., Kaner E., Meier P., Michie S. Associations between socio-economic factors and alcohol consumption: A population survey of adults in England. PLoS ONE. 2019;14:e0209442. doi: 10.1371/journal.pone.0209442. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Sudhinaraset M., Wigglesworth C., Takeuchi D.T. Social and cultural contexts of alcohol use: Influences in a social–ecological framework. Alcohol Res. Curr. Rev. 2016;38:35–45. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;88:105906. doi: 10.1136/bmj.n71. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. [(accessed on 23 February 2022)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools .
- 33. Morrison C.N., Byrnes H.F., Miller B.A., Wiehe S.E., Ponicki W.R., Wiebe D.J. Exposure to alcohol outlets, alcohol access, and alcohol consumption among adolescents. Drug Alcohol Depend. 2019;205:107622. doi: 10.1016/j.drugalcdep.2019.107622. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Seid A.K., Berg-Beckhoff G., Stock C., Bloomfield K. Is proximity to alcohol outlets associated with alcohol consumption and alcohol-related harm in Denmark? Nord. Stud. Alcohol Drugs. 2018;35:118–130. doi: 10.1177/1455072518759829. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Lamb K.E., Thornton L.E., Teychenne M., Milte C., Cerin E., Ball K. Associations between access to alcohol outlets and alcohol intake and depressive symptoms in women from socioeconomically disadvantaged neighbourhoods in Australia. BMC Public Health. 2017;17:83. doi: 10.1186/s12889-017-4022-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Toornstra A., Massar K., Hurks P.P.M., Timmermans M.M.M.S., Kok G., Curfs L.M.G. Perceptions of Alcohol and Alcohol Use among Community Members and Young Adults in Ukraine. Subst. Use Misuse. 2020;55:1269–1279. doi: 10.1080/10826084.2020.1735436. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Martins J.G., Guimarães M.O., Jorge K.O., Silva C.J.D.P., Ferreira R.C., Pordeus I.A., Kawachi I., Zarzar P.M.P.D.A. Binge drinking, alcohol outlet density and associated factors: A multilevel analysis among adolescents in Belo Horizonte, Minas Gerais. State Brazil. 2020;36:e00052119. doi: 10.1590/0102-311x00052119. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Foster S., Trapp G., Hooper P., Oddy W.H., Wood L., Knuiman M. Liquor landscapes: Does access to alcohol outlets influence alcohol consumption in young adults? Health Place. 2017;45:17–23. doi: 10.1016/j.healthplace.2017.02.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Foster S., Hooper P., Divitini M., Knuiman M., Trapp G. Over the limit? Testing non-linear associations between alcohol outlets and young adults’ alcohol consumption. Drug Alcohol Rev. 2020;39:664–670. doi: 10.1111/dar.13115. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Auchincloss A.H., Niamatullah S., Adams M., Melly S.J., Li J., Lazo M. Alcohol outlets and alcohol consumption in changing environments: Prevalence and changes over time. Subst. Abus. Treat. Prev. Policy. 2022;17:1–12. doi: 10.1186/s13011-021-00430-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Cardoza L.S., MacHado C.O., Santos C.T.d., Höfelmann D.A. Alcohol outlets availability in school neighborhoods and alcohol use among adolescents. Cad. Saude Publica. 2020;36:e00062919. doi: 10.1590/0102-311x00062919. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Mair C., Sumetsky N., Gruenewald P.J., Lee J.P. Microecological Relationships Between Area Income, Off-Premise Alcohol Outlet Density, Drinking Patterns, and Alcohol Use Disorders: The East Bay Neighborhoods Study. Alcohol. Clin. Exp. Res. 2020;44:1636–1645. doi: 10.1111/acer.14387. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Kim M.J., Mason W.A., Herrenkohl T.I., Catalano R.F., Toumbourou J., Hill K. Influence of Early Onset of Alcohol Use on the Development of Adolescent Alcohol Problems: A Longitudinal Binational Study. Prev. Sci. 2017;18:1–11. doi: 10.1007/s11121-016-0710-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Staff J., Maggs J.L. Parents Allowing Drinking Is Associated With Adolescents’ Heavy Alcohol Use. Alcohol. Clin. Exp. Res. 2020;44:188–195. doi: 10.1111/acer.14224. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Soundararajan S., Narayanan G., Agrawal A., Prabhakaran D., Murthy P. Relation between age at first alcohol drink & adult life drinking patterns in alcohol-dependent patients. Indian J. Med. Res. 2017;146:606–611. doi: 10.4103/ijmr.IJMR_1363_15. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Plenty S.M., Evans-Whipp T.J., Chan G.C.K., Kelly A.B., Toumbourou J.W., Patton G.C., Hemphill S.A., Smith R. Predicting Alcohol Misuse Among Australian 19-Year-Olds from Adolescent Drinking Trajectories. Subst. Use Misuse. 2019;54:247–256. doi: 10.1080/10826084.2018.1517172. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Listabarth S., Vyssoki B., Waldhoer T., Gmeiner A., Vyssoki S., Wippel A., Blüml V., Gruber M., König D. Hazardous alcohol consumption among older adults: A comprehensive and multi-national analysis of predictive factors in 13,351 individuals. Eur. Psychiatry. 2020;64:1–21. doi: 10.1192/j.eurpsy.2020.112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Chaiyasong S., Huckle T., Mackintosh A.-M., Meier P., Parry C.D.H., Callinan S., Cuong P.V., Kazantseva E., Gray-Phillip G., Parker K., et al. Drinking patterns vary by gender, age and country-level income: Cross-country analysis of the International Alcohol Control Study. Drug Alcohol Rev. 2018;37:S53–S62. doi: 10.1111/dar.12820. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Cheah Y.K., Rasiah R. Analysis of the Determinants of Alcohol Consumption among Adult Males in Malaysia. J. Health Manag. 2017;19:28–38. doi: 10.1177/0972063416682548. [ DOI ] [ Google Scholar ]
- 50. Aguilar M.P.O., Palomera P.R., Núñez C.L., Maroño C.T., Gallegos S.V., Cabrera N.J.P., Deus J.E.R. The Role of Age of Onset in Problematic Alcohol Consumption: Artefact or Cohort Effect? Clin. Salud. 2022;33:11–17. doi: 10.5093/clysa2021a11. [ DOI ] [ Google Scholar ]
- 51. Islam M. Exploring the relationship between age at first drink, low-risk drinking knowledge and drinks counting: Six rounds of a country-wide survey in Australia. Public Health. 2020;179:160–168. doi: 10.1016/j.puhe.2019.10.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Aiken A., Clare P.J., Wadolowski M., Hutchinson D., Najman J.M., Slade T., Bruno R., McBride N., Kypri K., Mattick R.P. Age of Alcohol Initiation and Progression to Binge Drinking in Adolescence: A Prospective Cohort Study. Alcohol. Clin. Exp. Res. 2018;42:100–110. doi: 10.1111/acer.13525. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Mathialagan S., Tan C., Teng Y. Relationship between Parenting Styles and Alcohol Consumption among College Students In Segi College Subang Jaya, Malaysia. J. Psikol. Malays. 2017;31:96–101. [ Google Scholar ]
- 54. Dickens D.D., Jackman D.M., Stanley L.R., Swaim R.C., Chavez E.L. Alcohol consumption among rural African American and White adolescents: The role of religion, parents, and peers. J. Ethn. Subst. Abus. 2018;17:273–290. doi: 10.1080/15332640.2016.1179155. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Boua P.R., Soo C.C., Debpuur C., Maposa I., Nkoana S., Mohamed S.F., Choma S., Oduro A., Asiki G., Micklesfield L.K., et al. Prevalence and socio-demographic correlates of tobacco and alcohol use in four sub-Saharan African countries: A cross-sectional study of middle-aged adults. BMC Public Health. 2021;21:1126. doi: 10.1186/s12889-021-11084-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Šumskas L., Zaborskis A. Family Social Environment and Parenting Predictors of Alcohol Use among Adolescents in Lithuania. Int. J. Environ. Res. Public Health. 2017;14:1037. doi: 10.3390/ijerph14091037. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Haeny A.M., Gueorguieva R., Morean M.E., Krishnan-Sarin S., DeMartini K.S., Pearlson G.D., Anticevic A., Krystal J.H., O’Malley S.S. The Association of Impulsivity and Family History of Alcohol Use Disorder on Alcohol Use and Consequences. Alcohol. Clin. Exp. Res. 2020;44:159–167. doi: 10.1111/acer.14230. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Berent D., Wojnar M. Does Parental Alcohol Use Influence Children’s Age at First Alcohol Intake? A Retrospective Study of Patients with Alcohol Dependence. Healthcare. 2021;9:841. doi: 10.3390/healthcare9070841. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Gitatui M., Kimani S., Muniu S., Okube O. Determinants of harmful use of alcohol among urban slum dwelling adults in Kenya. Afr. Health Sci. 2019;19:2906–2925. doi: 10.4314/ahs.v19i4.12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. E Talley A., Hughes M.L., Wilsnack S.C., Hughes T.L. Women’s Self-Perceived Similarity to Their Mother and Associations with Patterns of Alcohol Misuse over 20 Years. Alcohol Alcohol. 2018;53:707–715. doi: 10.1093/alcalc/agy059. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Clare P.J., Aiken A., Yuen W.S., Peacock A., Boland V., Wadolowski M., Hutchinson D., Najman J., Slade T., Bruno R., et al. Parental supply of alcohol as a predictor of adolescent alcohol consumption patterns: A prospective cohort. Drug Alcohol Depend. 2019;204:107529. doi: 10.1016/j.drugalcdep.2019.06.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Najafi F., Hajizadeh M., Pasdar Y., Salimi Y., Hamzeh B., Matin B.K., Piroozi B., Rezaei S. Socioeconomic inequalities in tobacco, alcohol and illicit drug use: Evidence from Iranian Kurds. East. Mediterr. Health J. 2020;26:1294–1302. doi: 10.26719/emhj.20.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Čihák J. The effect of economic conditions on alcohol consumption. Int. Rev. Econ. 2020;67:481–497. doi: 10.1007/s12232-020-00351-z. [ DOI ] [ Google Scholar ]
- 64. Khan J., Shaw S. Socio-economic context of alcohol consumption and the associated risky behavior among male teenagers and young adults in India. J. Subst. Use. 2021;23:1–8. doi: 10.1080/14659891.2021.2006344. [ DOI ] [ Google Scholar ]
- 65. Baena B.C., Meneses C., Caperos J.M., Prieto M., Uroz J. The Role of Religion and Religiosity in Alcohol Consumption in Adolescents in Spain. J. Relig. Health. 2019;58:1477–1487. doi: 10.1007/s10943-018-0694-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Bai Z. Does frequent prayer help reduce alcohol use? Heterogeneity in religious contexts and drinking styles. Ment. Health Relig. Cult. 2021;24:151–163. doi: 10.1080/13674676.2020.1826915. [ DOI ] [ Google Scholar ]
- 67. Yockey R.A., King K.A., Vidourek R.A. The Epidemiology of Recent Alcohol Use Among a National Sample of Middle Eastern College Students. J. Drug Educ. 2020;49:30–42. doi: 10.1177/0047237920929328. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. McAuslan P., Altairi S., Siefert C. The Relationship between Religion, Gender, and Substance Use in Arab American Muslim Emerging Adults. J. Muslim Ment. Health. 2020;14:63–86. doi: 10.3998/jmmh.10381607.0014.203. [ DOI ] [ Google Scholar ]
- 69. Stauner N., Exline J.J., Kusina J.R., Pargament K.I. Religious and spiritual struggles, religiousness, and alcohol problems among undergraduates. J. Prev. Interv. Community. 2019;47:243–258. doi: 10.1080/10852352.2019.1603678. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Rivera C.J., Lauger T.R., A Cretacci M. Religiosity, Marijuana Use, and Binge Drinking: A Test of the Moral Community Hypothesis. Sociol. Relig. 2018;79:356–378. doi: 10.1093/socrel/srx071. [ DOI ] [ Google Scholar ]
- 71. Agrawal A., Grant J.D., Haber J.R., Madden P.A., Heath A.C., Bucholz K.K., Sartor C.E. Differences between White and Black young women in the relationship between religious service attendance and alcohol involvement. Am. J. Addict. 2017;26:437–445. doi: 10.1111/ajad.12462. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Kathol N., Sgoutas-Emch S. Alcohol Use in College: The Relationship between Religion, Spirituality, and Proscriptive Attitudes Toward Alcohol. J. Relig. Health. 2017;56:437–449. doi: 10.1007/s10943-016-0210-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Thompson W.E. Social Support, Religious Involvement and Alcohol Use among Students at a Conservative Religious University. Behav. Sci. 2017;7:34. doi: 10.3390/bs7020034. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Guo S., Metcalfe C. Religion as a Social Control: A Longitudinal Study of Religious Involvement and Substance Use. Crime Delinquency. 2019;65:1149–1181. doi: 10.1177/0011128718787510. [ DOI ] [ Google Scholar ]
- 75. Jager J., Putnick D., Bornstein M.H., II More Than Just Convenient: The Scientific Merits of Homogeneous Convenience Samples. Monogr. Soc. Res. Child Dev. 2017;82:13–30. doi: 10.1111/mono.12296. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Colchero M.A., Barrientos-Gutiérrez T., Guerrero-López C.M., Bautista-Arredondo S. Density of alcohol-selling outlets and prices are associated with frequent binge drinking in Mexico. Prev. Med. 2022;154:106921. doi: 10.1016/j.ypmed.2021.106921. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (878.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Research article
- Open access
- Published: 07 June 2023
Alcohol consumption and all-cause and cause-specific mortality among US adults: prospective cohort study
- Yalan Tian 1 ,
- Jiahui Liu 1 ,
- Yue Zhao 1 ,
- Nana Jiang 1 ,
- Xiao Liu 1 ,
- Gang Zhao 2 &
- Xia Wang 1
BMC Medicine volume 21 , Article number: 208 ( 2023 ) Cite this article
14k Accesses
12 Citations
111 Altmetric
Metrics details
A Research article to this article was published on 03 July 2023
Previous studies have shown inconsistent findings regarding the association of light to moderate alcohol consumption with cause-specific mortality. Therefore, this study sought to examine the prospective association of alcohol consumption with all-cause and cause-specific mortality in the US population.
This was a population-based cohort study of adults aged 18 years or older in the National Health Interview Survey (1997 to 2014) with linkage to the National Death Index records through December 31, 2019. Self-reported alcohol consumption was categorized into seven groups (lifetime abstainers; former infrequent or regular drinkers; and current infrequent, light, moderate, or heavy drinkers). The main outcome was all-cause and cause-specific mortality.
During an average follow-up of 12.65 years, among the 918,529 participants (mean age 46.1 years; 48.0% male), 141,512 adults died from all causes, 43,979 from cardiovascular disease (CVD), 33,222 from cancer, 8246 from chronic lower respiratory tract diseases, 5572 from accidents (unintentional injuries), 4776 from Alzheimer’s disease, 4845 from diabetes mellitus, 2815 from influenza and pneumonia, and 2692 from nephritis, nephrotic syndrome, or nephrosis. Compared with lifetime abstainers, current infrequent, light, or moderate drinkers were at a lower risk of mortality from all causes [infrequent—hazard ratio: 0.87; 95% confidence interval: 0.84 to 0.90; light: 0.77; 0.75 to 0.79; moderate 0.82; 0.80 to 0.85], CVD, chronic lower respiratory tract diseases, Alzheimer’s disease, and influenza and pneumonia. Also, light or moderate drinkers were associated with lower risk of mortality from diabetes mellitus and nephritis, nephrotic syndrome, or nephrosis. In contrast, heavy drinkers had a significantly higher risk of mortality from all causes, cancer, and accidents (unintentional injuries). Furthermore, binge drinking ≥ 1 day/week was associated with a higher risk of mortality from all causes (1.15; 1.09 to 1.22), cancer (1.22; 1.10 to 1.35), and accidents (unintentional injuries) (1.39; 1.11 to 1.74).
Conclusions
Infrequent, light, and moderate alcohol consumption were inversely associated with mortality from all causes, CVD, chronic lower respiratory tract diseases, Alzheimer’s disease, and influenza and pneumonia. Light or moderate alcohol consumption might also have a beneficial effect on mortality from diabetes mellitus and nephritis, nephrotic syndrome, or nephrosis. However, heavy or binge had a higher risk of all-cause, cancer, and accidents (unintentional injuries) mortality.
Peer Review reports
Alcohol is widely consumed in the United States and worldwide. The possible beneficial and detrimental effects of alcohol consumption, as investigated in many studies, have been hotly debated [ 1 , 2 ]. Alcohol consumption has been linked to a range of health and social consequences [ 2 ]. Many studies have examined the association between alcohol consumption and all-cause, cardiovascular disease (CVD), and cancer mortality but with inconsistent findings [ 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. In addition, most studies did not separately explore the specific types of other-causes mortality related to alcohol consumption, with the exception of all-cause, CVD, and cancer mortality [ 12 , 13 , 14 ]. Therefore, it is crucial to further confirm these previous research results as well as identify and clarify new relationships between alcohol consumption and disease/injury to inform policy efforts and prevention programs.
The harm caused by alcohol accounts for about one tenth of the total health impact of alcohol (9.9% and 12.6% in low- and high-income countries, respectively) [ 15 ]. The literature has reported that alcohol consumption is associated with a higher risk of injuries, mainly based on cross-sectional [ 16 , 17 , 18 , 19 ], case–crossover [ 20 , 21 ], and case–control studies [ 22 ]. The magnitude of the effects in the alcohol and injury relationship may be impacted by the study design. Few prospective cohort studies have explored the risk of accidents (unintentional injuries) mortality due to drinking.
Some studies found that light or moderate alcohol consumption is associated with a lower risk of diabetes mellitus [ 23 , 24 , 25 ]. However, one meta-analysis showed that the reductions in risk were attenuated if former drinkers were excluded from the reference category [ 3 ]. Many studies may overestimate the degree of risk reduction for moderate drinkers as a result of comparing drinkers to a less healthy nondrinking reference category [ 26 ]. Furthermore, the association between alcohol consumption and the respiratory system remains controversial. A large population-based study in men found that alcohol use had a lower risk of death from overall respiratory disease and obstructive pulmonary disease (COPD) for all drinkers, but the study did not distinguish between moderate and occasional drinking [ 27 ]. Nevertheless, another study in individuals aged 12–41 years indicated that alcohol consumption had a U-shaped association with risk of new-onset asthma [ 28 ].
Few studies have examined the association between alcohol consumption and mortality due to Alzheimer’s disease. A Norwegian study examined the relationship between alcohol consumption and risk of dementia-related death, but the participants only comprised people aged between 60 and 80 years [ 29 ]. Some studies focused on the risk of alcohol-related dementia and cognitive decline, but they did not estimate the outcome of death due to Alzheimer’s disease [ 30 , 31 ]. Additionally, several studies reported that alcohol consumption was related to chronic kidney disease [ 32 , 33 ]. In contrast, one study found that alcohol consumption was not associated with an increased risk of renal dysfunction [ 34 ]. At present, no study has estimated the association between alcohol consumption and mortality from nephritis, nephrotic syndrome, or nephrosis.
The most recent national data related to alcohol consumption are available from the National Health Interview Survey (NHIS). Also, the National Center for Health Statistics (NCHS) has recently updated their information on the NHIS surveys linked to the National Death Index (NDI) data through December 31, 2019. Two previous studies on the NHIS [ 12 , 13 ] only examined that the association of alcohol consumption with all-cause, CVD, and cancer mortality and current infrequent drinkers were also not separate from current light drinkers or lifetime abstainers. Therefore, we comprehensively estimated the recent association of alcohol consumption with all-cause and cause-specific mortality among a nationally representative sample of US adults.
Study population
The NHIS is a multi-purpose health survey of the civilian, noninstitutionalized, household population of the US continuously conducted by the NCHS and Centers for Disease Control and Prevention since 1957. A stratified, multistage probability sample design is used to represent the civilian noninstitutionalized US population. The NHIS data were collected through computer-assisted personal interviews administered by interviewers directed and trained by the US Census Bureau.
Information on basic health status was collected for all household members. One randomly sampled adult from each household was thoroughly interviewed about health and lifestyle behaviors, including health status, health behaviors, and healthcare utilization. Information on the study design, methodology, and weights is described in detail elsewhere [ 35 ].
This study used data from NHIS years that included alcohol consumption data from 1997 to 2014, with linkage to the NDI through December 31, 2019. All data obtained from the survey are publicly available on-line via the NCHS website [ 36 ]. We included participants aged 18 years and older at baseline with mortality follow-up information, including underlying cause of death.
The assessment of study exposure
The questionnaires relating to alcohol use status and patterns of consumption were administered for all sample adults. Alcohol-related items in NHIS included the following: (1) In any 1 year, have you had at least 12 drinks of any type of alcoholic beverage? (2) In your entire life, have you had at least 12 drinks of any type of alcoholic beverage? (3) In the past year, how often did you drink any type of alcoholic beverage? (4) In the past year, on those days that you drank alcoholic beverages, on the average, how many drinks did you have? (5) In the past year, on how many days did you have 5 or more/4 or more drinks of any alcoholic beverage?
Based on the responses to questions about drinking alcoholic beverages, participants were categorized into seven alcohol consumption groups [ 37 ]: lifetime abstainer (< 12 drinks in one’s lifetime), former infrequent drinker (< 12 drinks in any previous year and none in the past year), former regular drinker (≥ 12 drinks in any previous year in one’s lifetime but none in the past year), current infrequent drinker (1–11 drinks in the past year), current light drinker (≥ 12 drinks in the past year but ≤ 3 drinks/week), current moderate drinker (> 3 drinks/week to ≤ 7 drinks/week for women and > 3 drinks/week to ≤ 14 drinks/week for men), and current heavy drinker (> 7 drinks/week for women and > 14 drinks/week for men). Data about binge drinking status was collected by using the responses to questions. The answers using unit as days per year were transferred into using unit as days per week (or month). One alcoholic drink-equivalent is defined as one that contains 14 g of pure alcohol (about 0.6 fluid ounces or 1.2 tablespoons), as is found in one 12 oz of beer (5% alcohol), one 5 oz glass of wine (12% alcohol), or one 1.5 oz shot of distilled spirits (40% alcohol) [ 37 ].
To avoid drinker misclassification errors, current infrequent drinkers were separate from current light drinkers or lifetime abstainers. No drinks in past year was classified as former drinkers in data years 1997 to 2000, which did not make a distinction between former infrequent and former regular drinkers. Former drinkers in data years 1997 to 2000 were classified as former regular drinkers. Thus, former infrequent drinkers in data years 1997 to 2000 were missing. Participants classified as “former unknown frequency drinker,” “current unknown level drinker,” or “drinking status unknown” were not included in this study.
Mortality ascertainment
Linked mortality files provided mortality follow-up data from the month of the interview through December 31, 2019. Mortality information was obtained using a probabilistic match algorithm between the NHIS surveys and death certificate records in the NDI data [ 38 ]. All NHIS participants aged 18 years and older with sufficient identifying data were eligible for mortality follow-up. To reduce participant disclosure risk, the NCHS developed data-perturbation techniques. Information regarding participant vital status was not perturbed. Previous studies have confirmed the accuracy of information on mortality in the NDI records [ 39 ].
For the 1997–2014 NHIS, the cause-specific death categories included 9 groups selected from the 113 NCHS underlying cause-of-death recodes, whereas those of 2015–2018 NHIS only included 5 groups. Thus, data from the 2015–2018 NHIS were not included in this study. Underlying cause of death were coded using the 9th Revision International Statistical Classification of Diseases (ICD-9) through 1998, and the remainder for case definition was used according to the 10th Revision (ICD-10) from 1999 to the present. Considering changes between the 2 coding systems, causes of deaths occurring before 1999 were converted into comparable ICD-10–based underlying-cause-of-death groups [ 40 ]. The study outcomes were all-cause and cause-specific mortality (CVD, cancer, chronic lower respiratory tract diseases, accidents and injuries, Alzheimer’s disease, diabetes mellitus, influenza and pneumonia, and nephritis, nephrotic syndrome, or nephrosis). Please see the additional file for the ICD-10 codes (see Additional file 1 : Table S1).
A total of 1,345,653 NHIS participants 18 years and older during 1997 to 2014 were included in the study. Among these, 427,124 were excluded due to participants with former unknown frequency, current unknown level, and drinking status unknown ( n = 17,903), missing mortality follow-up information ( n = 406,699), or missing data information on potential covariates ( n = 2522), leading to a final analytical sample of 918,529 adults. Please see flow chart of the selection of study participants (see Additional file 1 : Figure S1).
Study covariates
A set of covariates available in all NHIS surveys was included as confounders to estimate alcohol consumption. Demographic characteristics included age, sex, race/ethnicity, education, and marital status. Lifestyle factors included body mass index, physical activity, and smoking status. Chronic health conditions included cancer, diabetes mellitus, heart disease, hypertension, stroke, asthma, emphysema, and chronic bronchitis.
Body mass index was calculated using self-reported height and weight as weight in kilograms divided by height in meters squared. Smoking status was estimated in the NHIS Sample Adult Files. Smoking was defined as three categories—never smoker, former smoker, and current smoker—using self-reported responses to the survey questions about smoking. Never smokers were defined as those who reported smoking fewer than 100 cigarettes in their entire life. Current smokers were defined as those who reported smoking at least 100 cigarettes in their life and were currently smoking every day or some days. Former smokers were defined as those who smoked more than 100 cigarettes during their lifetime but currently did not smoke at all.
In a separate part of the questionnaires, questions about physical activity were asked as part of the NHIS periodic adult prevention module. Participants were divided into three groups according to self-reported physical activity: high, moderate, and low levels of physical activity. Participants were defined as having a low level of physical activity if they never engaged in or were unable to engage in such activities for at least 10 min. Participants were defined as having a moderate level of physical activity if they engaged in moderate-intensity activity at least 5 times a week for at least 30 min per day, or both, meeting the criteria of physical activity guidelines [ 41 ]. Participants were defined as having a high level of physical activity if they performed vigorous-intensity activity 3 or more days per week for at least 20 min per day. As health status was determined in the family core interview, it may have been proxy-reported. Hypertension, heart disease, stroke, cancer, diabetes, asthma, emphysema, and chronic bronchitis were defined by using participants’ self-reported responses to physician diagnoses.
Statistical analyses
All analyses were done to account for the complex, stratified, multistage cluster sampling design of the NHIS by using stratification, clustering, and sample weights in the NHIS data. The baseline characteristics of participants are obtained at the start of survey. To compare baseline characteristics among different groups, we used the χ 2 test for categorical variables and analysis variance for continuous variables. Also, we calculated the distribution of alcohol consumption across different years from 1997 to 2014.
For primary analyses, we set lifetime abstainers as the reference group. We examined whether there was any association between alcohol consumption and mortality due to CVD, cancer, chronic lower respiratory disease (e.g., asthma, bronchitis, and emphysema), accidents (unintentional injuries), Alzheimer’s disease, diabetes mellitus, pneumonia and influenza, nephritis, nephrotic syndrome, or nephrosis, or any causes by using Cox proportional-hazards regression models to calculate the hazard ratio (HR) and 95% confidence intervals (CIs). With Cox regression, the influence of multiple predictors on the hazard, that is, risk of mortality, can be modeled. The model relies on two critical assumptions: the proportional hazards and the log-linearity of covariates. No violations to the proportional hazards assumption were found using Schoenfeld residual diagram. The data set contains no outliers. The above-mentioned associations were investigated by adjusting for the following covariates: age, sex, race, or ethnicity (model 1); model 1 plus education level, physical activity, body mass index, smoking status, hypertension, heart disease, stroke, cancer, diabetes, asthma, emphysema, or chronic bronchitis in a separate model (model 2). Model assumptions were checked for all the analyses.
Additionally, we performed the dose–response analysis to quantitatively estimate the association of current alcohol consumption (as a continuous variable) with all-cause and cause-specific mortality. A potential curve linear relation was assessed by using restricted cubic splines with four knots at the 5th, 35th, 65th, and 95th percentiles of the distribution [ 42 ]. The non-linear association of alcohol consumption with all-cause and cause-specific mortality using restricted cubic splines models [ 43 ]. Non-linearity was detected for using the likelihood ratio test to compare two models: one containing only a linear effect and the other also containing cubic spline terms [ 44 ].
Sensitivity analyses were conducted to confirm the findings of this study. First, we recalculated the estimates by excluding participants who died within the first 2 years (i.e., a 2-year lag). Second, we conducted the analyses by excluding individuals with physician-diagnosed diseases. Third, to examine the effects of missing data, a sensitivity analysis was conducted after multiple imputations for variables with missing values. The Markov chain Monte Carlo imputation assumes a normal distribution for the variables in the imputation model [ 45 ]. Reliable estimates can be obtained even when the distribution of variables is not normal. Fourth, to examine if there is abstainer biases, we recalculated HR estimates by using current infrequent drinkers as reference groups instead of abstainers as the reference group. Fifth, we estimated HRs for all-cause mortality according to drinking status in the individual NHIS survey, and then performed pooled analyses to obtain the summary estimates across survey years.
Furthermore, we performed stratified analyses to estimate whether the relationship between alcohol consumption and mortality varied by age (< 60 vs . ≥ 60), sex, race or ethnicity (white vs. non-white), and smoking status (never smoked vs. ever smoked). In addition, according to binge-drinking status, the participants were divided into 5 subgroups: lifetime abstainer, no binge drinking, binge drinking < 1 day/month, binge drinking < 1 day/week, and binge drinking ≥ 1 day/week. Cox proportional-hazards regression models were used to estimate the effect of binge drinking on all-cause and cause-specific mortality.
Stata software (version 15.1) was used for dose–response analysis. All other statistical analyses were performed using survey modules of SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA). All P values were based on two-tailed testing, and P values less than 0.05 denoted statistical significance.
Population characteristics
Among the 918,529 eligible adults in the NHIS (mean age 46.1 years, 52.0% women) in this study, 24.4% ( n = 223,757) were lifetime abstainers at baseline in 1997, 6.8% ( n = 62,185) were former infrequent drinkers, 8.6% ( n = 78,769) were former regular drinkers, 13.3% ( n = 122,441) were current infrequent drinkers, 28.8% ( n = 264,067) were current light drinkers, 13.4% ( n = 122,825) were current moderate drinkers, and 4.8% ( n = 44,485) were current heavy drinkers. Compared with lifetime abstainers, current heavy drinkers were more likely to be young, men, non-Hispanic whites, current smokers, and unmarried, and have high levels of physical activity, greater than high school education, and normal weight; they were also more likely to have cancer but less likely to have diabetes, hypertension, and CVD at baseline. Table 1 presents the baseline characteristics of the participants according to alcohol consumption status. Also, this study showed that the distribution of alcohol consumption was consistent across different years from 1997 to 2014 (see Additional file 1 : Table S2).
During 11,650,593 person-years of follow-up (an average follow-up of 12.65 years and a maximum follow-up of 22.75 years), 141,512 participants died from all causes, 43,979 from CVD, 33,222 from cancer, 8246 from chronic lower respiratory tract diseases, 5572 from accidents (unintentional injuries), 4776 from Alzheimer’s disease, 4845 from diabetes mellitus, 2815 from influenza and pneumonia, and 2692 from nephritis, nephrotic syndrome, or nephrosis.
Alcohol consumption and all-cause and cause-specific mortality
Table 2 shows adjusted HRs for all-cause and cause-specific mortality according to alcohol consumption status at baseline. In the fully adjusted model (model 2), compared with lifetime abstainers, current infrequent, light, and moderate drinkers had a lower risk of all-cause mortality, with HRs of 0.87 (95% CI: 0.84 to 0.90), 0.77 (0.75 to 0.79), and 0.82 (0.80 to 0.85), respectively. Similarly, current infrequent, light, or moderate drinkers were associated with a reduced risk of mortality from CVD (infrequent: 0.86; 0.82 to 0.91; light: 0.76; 0.73 to 0.80; moderate: 0.78; 0.74 to 0.82), from chronic lower respiratory tract diseases (infrequent: 0.88; 0.77 to 0.99; light: 0.68; 0.60 to 0.76; moderate: 0.78; 0.68 to 0.90), from Alzheimer’s disease (infrequent: 0.76; 0.65 to 0.88; light: 0.68; 0.59 to 0.78; moderate: 0.83; 0.69 to 0.99), and from influenza and pneumonia (infrequent: 0.69; 0.57 to 0.83; light: 0.63; 0.52 to 0.75; moderate: 0.58; 0.46 to 0.73). This study also found that light or moderate drinkers had a reduced risk of mortality from diabetes mellitus (light: 0.72; 0.61 to 0.84; moderate 0.73; 0.60 to 0.88) and nephritis, nephrotic syndrome, or nephrosis (light: 0.66; 0.54 to 0.81; moderate 0.62; 0.48 to 0.79).
However, heavy drinkers had a higher risk of mortality from all causes (1.07; 1.03 to 1.12) and accidents (unintentional injuries) (1.48; 1.22 to 1.80). Although light drinkers were associated with a reduced risk of mortality from cancer (0.86; 0.81 to 0.91), heavy drinkers had an obviously higher risk of mortality from cancer (1.24; 1.14 to 1.34).
Dose–response analysis
This study performed a dose–response analysis between current alcohol consumption (as a continuous variable) and all-cause and cause-specific mortality. Figure 1 and Additional file 1 (Figures S2–S9) illustrate that a nonlinear association of current alcohol consumption with all-cause and cause-specific mortality (all p < 0.05 for the nonlinear test). Figure 1 indicates that alcohol consumption had a J-shaped association with risk of all-cause mortality. These findings were corresponding to those when current alcohol consumption was regarded as a category variable (lifetime abstainers, and current infrequent, light, moderate, and heavy drinkers) in Table 2 .
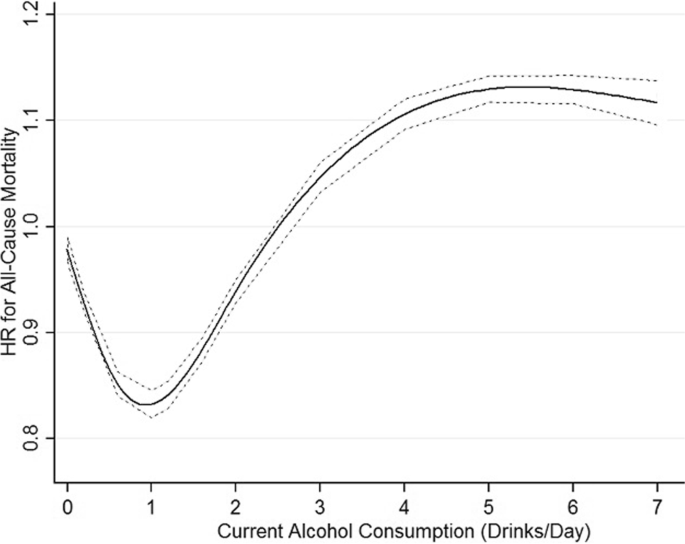
Dose–response relationship between alcohol consumption and risk of mortality from all causes. A nonlinear relationship of current alcohol consumption (as a continuous variable) with all-cause mortality ( p < 0.05 for the nonlinear test), using maximally adjusted estimates (adjusted for sex, age, race/ethnicity, education, marital status, body mass index, physical activity, smoking, and physician-diagnosed diseases (heart disease, stroke, cancer, diabetes, hypertension, asthma, emphysema, and chronic bronchitis). That indicates that alcohol consumption had a J-shaped association with risk of all-cause mortality. HR, hazard ratio
Sensitivity analyses
We performed the 2-year lag analysis to remove the effect of early deaths on the results. Further excluding early deaths in the 2-year lag analysis had little effect on the observed summary estimates (Table 2 ). Also, we analyzed again the dataset after multiple imputations for all variables with missing values, and similar results were obtained (see Additional file 1 : Table S3).
In addition, we calculate HR estimates by using current infrequent drinkers as reference groups (see Additional file 1 : Table S4). We still observed favorable effects of current light or moderate drinking on mortality from all causes, CVD, diabetes mellitus, and nephritis, nephrotic syndrome, or nephrosis. The harmful effects of heavy drinking on mortality from all causes, cancer, and accidents (unintentional injuries) seem to be more evident.
Furthermore, we estimated HRs for all-cause mortality according to drinking status in the individual NHIS survey, and then performed pooled analyses to obtain the summary estimates across survey years (see Additional file 1 : Table S5). The results were in agreement with those in primary analyses. To further confirm the robustness of our findings, we recalculated the risk estimates by excluding participants with physician-diagnosed diseases (see Additional file 1 : Table S6). The positive associations between former regular drinking and all-cause, CVD, cancer, and diabetes mellitus mortality seen previously were not observed. The negative associations between current infrequent drinking and mortality from lower respiratory tract diseases, Alzheimer’s disease, and influenza and pneumonia and between current moderate drinking and mortality from diabetes mellitus and nephritis, nephrotic syndrome, or nephrosis also disappeared. In general, the impact of excluding participants with physician-diagnosed diseases on the estimates of other associations was small.
Stratification analysis
Stratified analyses by sex, age, race, and smoke status were conducted to further verify the findings of this study (see Additional file 1 : Table S7). The results showed that the associations between alcohol consumption and mortality risk varied with sex, age, race, and smoke status. A protective effect of light or moderate drinking on mortality was more pronounced in women than in men. In contrast, current heavy drinkers had a higher risk of all-cause and cancer mortality in men but not in women.
Favorable effects of current infrequent, light, or moderate drinking on mortality from Alzheimer’s disease were observed in participants older than 60 but not in those younger than 60. Beneficial effects of moderate drinking on mortality from all causes, CVD, cancer, chronic lower respiratory tract diseases, Alzheimer’s disease, and diabetes mellitus were found in non-Hispanic white subjects but not in other ethnic groups.
Furthermore, the protective effect of light or moderate drinking on mortality, particularly mortality from influenza and pneumonia, was more obvious in never smokers than in those who ever smoked. Meanwhile, the detrimental effect of heavy drinking on mortality from all causes, cancer, and accidents (unintentional injuries) was also more pronounced in those who had ever smoked than in never smokers.
Binge drinking and all-cause and cause-specific mortality
Compared with lifetime abstainers, participants with binge drinking ≥ 1 day/week were associated with an increased risk of mortality from all causes (1.15; 1.09 to 1.22), cancer (1.22; 1.10 to 1.35), and accidents (unintentional injuries) (1.39; 1.11 to 1.74) in the multivariable adjustment model but were not associated with the risk of mortality from CVD, chronic lower respiratory tract diseases, Alzheimer’s disease, diabetes mellitus, influenza and pneumonia, and nephritis, nephrotic syndrome, or nephrosis. The associations were not significant changed for all-cause and cause-specific mortality in the 2-year lag analysis (Table 3 ).
In this nationally representative cohort study, we found that infrequent, light, or moderate alcohol consumption were associated with a lower risk of mortality from all causes, CVD, chronic lower respiratory tract diseases, Alzheimer’s disease, and influenza and pneumonia, whereas heavy or binge drinking were associated with a significantly higher risk of mortality from all causes, cancer, and accidents (unintentional injuries). Light or moderate alcohol consumption was also inversely associated with mortality from diabetes mellitus and nephritis, nephrotic syndrome, or nephrosis. The protective effect of light to moderate alcohol consumption was more pronounced in women, older populations, non-Hispanic white subjects, and never smokers, whereas the adverse effect of heavy drinking on mortality from all causes, cancer, and accidents (unintentional injuries) was obvious in younger age groups and those who ever smoked.
Alcohol use has a complex association with health. Our study suggested that heavy alcohol consumption was associated with a significantly higher risk of mortality from all causes, cancer, and accidents (unintentional injuries). The results are consistent with a recent study showed that there were the strong association of alcohol use with the risk of cancer and injuries [ 1 ]. These indicate that mortality from cancer and accidents (unintentional injuries) may be the main causes of death caused by alcohol consumption.
Our findings of the associations between alcohol consumption and all-cause, CVD, and cancer mortality were consistent with some [ 3 , 4 , 12 , 13 , 46 , 47 ], but not all [ 6 , 7 , 27 , 48 ] previous studies. Analyses of higher-quality studies free from abstainer biases found no evidence of reduced risk of mortality at low levels of alcohol consumption [ 6 ]. Some studies on alcohol and health may misclassify former and occasional drinkers as abstainers and place them in the reference group, which may underlie positive health outcomes observed in people with low alcohol consumption. Considering abstainer biases, former or current infrequent drinkers were not included in the “abstainer” reference group, which may provide more accurate information on the drinker category in the current study. Compared with the two previous studies on the NHIS [ 12 , 13 ], this study also included more recent data and three additional category groups: “former infrequent drinker,” “former regular drinker,” and “current infrequent drinker.” This study found beneficial effects of current infrequent alcohol consumption on all-cause and CVD mortality, which were not found in the two previous studies [ 12 , 13 ]. The current study has incorporated updated data from NHIS spanning from 1997 to 2014, along with mortality outcomes that were tracked until the end of 2019. In addition, this study examined the association between alcohol consumption and cause-specific mortality, such as mortality from chronic lower respiratory tract diseases, accidents (unintentional injuries), Alzheimer’s disease, diabetes mellitus, influenza and pneumonia, and nephritis, nephrotic syndrome, or nephrosis.
Stratified analyses by sex suggested that a protective effect of light or moderate drinking on mortality was lower in men than in women and current heavy drinkers had a higher risk of all-cause and cancer mortality in men but not in women in this study. One study in China found that alcohol consumption was associated with a lower risk of COPD mortality in men than in women [ 49 ]. Data from the nationwide China Kadoorie Biobank prospective study showed that men drink more than 20 times as much as women [ 50 ]. Genetic evidence shows that the apparently protective effects of moderate alcohol intake against stroke are not mainly caused by alcohol itself and are largely artifacts of reverse causation and confounding [ 50 ]. Alcohol drinking remains predominately a male phenomenon in China, and distilled spirits are the main type of alcoholic beverage, which is very different from that in most Western populations. The current study of the US population observed a protective effect of light drinking on mortality from chronic lower respiratory tract diseases. Previous studies also found an association between moderate drinking and a lower respiratory disease mortality rate [ 51 , 52 ]. A cohort study of participants aged 65 years or older found that occasional or moderate drinking was associated with a lower risk of death from all respiratory disease and COPD [ 53 ]. A cross-sectional study of men from Finland, Italy, and the Netherlands showed that moderate drinkers had lower COPD mortality relative to nondrinkers and heavy drinkers [ 54 ]. It is possible that mild concentrations of alcohol increase mucociliary clearance and bronchodilation and reduce the airway inflammation and injury found in asthma and COPD [ 55 ]. On the other hand, prolonged and heavy drinking may impair mucociliary clearance, worsen asthma, and most likely lead to lung-function decline and mortality in patients with COPD [ 55 ].
Most studies that estimate the risk of alcohol-related injuries primarily focus on data from hospital emergency departments [ 56 ], so the risk of alcohol-related injuries may be underestimated. In one study, the estimates of emergency department injury ranged from 5 to 40% by using emergency department data from 27 countries [ 57 ]. This prospective population-based study found that heavy drinkers had a 71% higher risk of mortality accidents (unintentional injuries) after excluding those with chronic diseases. A study indicated strong dose–response associations of amount of alcohol consumption in the past 3 h with odds of vehicle injury [ 58 ]. A meta-analysis showed that even moderate alcohol consumption roughly doubled the odds of injury and that the risks increased sharply at higher levels of alcohol intake [ 56 ]. Overall, this study concludes that actions to reduce the risk of death from accidents (unintentional injuries) associated with alcohol consumption, especially heavy drinking patterns, should be urgently strengthened.
This study found a favorable effect of current infrequent, light, or moderate drinking on mortality from Alzheimer’s disease in people older than 60 but not in those younger than 60. One study concluded that the risk of dementia-related death was significantly higher among elderly abstainers compared to individuals that drank alcohol [ 29 ], which was consistent with our findings. A Mendelian randomization study found a causal association between alcohol consumption and an earlier Alzheimer’s disease age of onset survival, but not between alcohol consumption and late-onset Alzheimer’s disease risk [ 59 ]. Two meta-analyses found that light to moderate alcohol consumption was associated with a 25–38% reduction in the risk of Alzheimer’s disease, vascular dementia, and all-cause dementia compared with abstainers [ 30 , 31 ]. Our findings indicated that current infrequent, light, or moderate drinkers had a 17–32% lower risk of mortality due to Alzheimer’s disease compared to lifetime abstainers. However, this study cannot determine the causal relationship between alcohol consumption and Alzheimer’s disease mortality. Light or moderate alcohol use is probably not healthy by itself, but it is a marker of other healthy habits. It is possible that light or moderate alcohol use is just something that healthy people do. The molecular mechanisms of Alzheimer’s disease are still not entirely clear.
The current study using the NHIS data strictly defined a non-drinking category, in which former drinkers were not included in the reference group. Our results suggested that light or moderate drinkers had a lower risk of diabetes mortality. One meta-analysis showed no reduction in the risk of type 2 diabetes at any level of alcohol consumption among men and reductions in risk among moderate alcohol drinkers being specific to women [ 60 ]. However, Joosten et al. studied 35,625 adults aged 20–70 years and found that moderate alcohol consumption was associated with an approximately 40% lower risk of type 2 diabetes compared with abstention, and they did not find differences between the two sexes [ 61 ]. Given that no clear conclusion has been reached, recommendations must be made cautiously, and the association between drinking alcohol and diabetes mortality is still a research topic.
Our study found that current infrequent, light, or moderate drinking were associated with a lower risk of mortality from influenza and pneumonia. A prospective Chinese elderly cohort study also found a lower risk of pneumonia mortality in occasional drinkers [ 53 ]. Clinical studies [ 62 , 63 ] have observed a beneficial effect of alcohol level on pneumonia rate in patients with traumatic brain injury, although the exact mechanism of the effect is unknown. However, one meta-analysis suggested an 83% increased risk of community-acquired pneumonia among adults who consumed some or high amounts of alcohol compared to those who consumed no or low amounts [ 64 ]. Thus, further research is needed to confirm the association between alcohol use and the risk of mortality from influenza and pneumonia.
This large cohort study found that light or moderate drinkers were associated with a lower risk of mortality from nephritis, nephrotic syndrome, or nephrosis. One study of an elderly Italian population suggested that moderate quantities of alcohol were not injurious to renal function [ 65 ]. Nevertheless, another study of rat models of acute and chronic progressive anti-thy1 glomerulonephritis suggested that moderate alcohol consumption might not bring specific protection in renal fibrotic disease [ 66 ]. Alcohol consumption has been related to the development or progression of chronic kidney disease [ 67 ]. A prospective population-based study found that moderate-heavy drinking was associated with elevated risk of albuminuria compared to light drinking [ 68 ]. It is likely that the effect of alcohol consumption on the risk of mortality from kidney diseases depends on the level of alcohol consumption. Nonetheless, due to the complexity of the pathogenesis of nephritis, nephrotic syndrome, or nephrosis, its causes and mechanism is still debatable. Therefore, more research is necessary to gain further knowledge on this topic.
We acknowledge that there are several limitations of the current study. First, as in other observational studies, our findings might be confounded by unidentified confounders that were not fully adjusted for, although there is relatively wide range of covariates available in NHIS. Second, the assessment of self-reported alcohol consumption in the NHIS was conducted at a single point in time, and it is possible that the research participants modified their consumption behavior during follow-up. Third, information on specific types of alcoholic beverages consumed was not collected uniformly. The effects of other components of each type of alcoholic drink besides ethanol on mortality risk cannot be fully ruled out. Fourth, the estimates may have been influenced by selection and misclassification biases of different participants; thus, we conducted sensitivity analyses by excluding participants who died in the first 2 years and those with physician-diagnosed diseases. Although the results of this study were basically consistent, these methods may not be sufficient to solve this problem. Fifth, due to stratifying participants by several obvious confounding factors and drinking habits simultaneously, the power may be insufficient to accurately estimate the risk of drinking, although the overall sample size is relatively large in the current study. Sixth, this study cannot distinguish mortality from specific cancer types. The findings of cancer mortality may not align with cause-specific cancer outcomes, such as liver cancer, oral cancer, and esophageal cancer. Also, the results related to cancer mortality may differ from the findings related to cancer incidence. Seventh, light or moderate drinking may provide some benefits in preventing deaths due to CVD, chronic lower respiratory tract diseases, Alzheimer’s disease, diabetes mellitus, influenza and pneumonia, and nephritis, nephrotic syndrome, or nephrosis, but this study cannot determine the causal relationship between alcohol consumption and those causes of death. Finally, the NHIS Linked Mortality File identified causes of death information by linkage to the NDI, which is derived from death certificates. Although this methodology has been previously validated by many published reports, the possibility of cause-of-death misclassification cannot be ruled out.
This large prospective study of US adults indicated that infrequent, light, or moderate drinking were associated with a lower risk of mortality from all causes, CVD, chronic lower respiratory tract diseases, Alzheimer’s disease, and influenza and pneumonia. Light or moderate drinking might have a protective effect on mortality from diabetes mellitus and nephritis, nephrotic syndrome, or nephrosis. However, heavy or binge drinking were associated with a significantly higher risk of all-cause, cancer, and accident (unintentional injuries) mortality. The deleterious effect of heavy alcohol consumption was apparent, although the beneficial effects of lower consumption were still observed. Therefore, recommending drinking must be done with caution.
Availability of data and materials
The NHIS data ( www.cdc.gov/nchs/nhis/index.htm ) are available to researchers upon application.
Abbreviations
Confidence interval
Obstructive pulmonary disease
- Cardiovascular disease
Hazard ratio
International Statistical Classification of Diseases, Injuries, and Causes of Death
National Center for Health Statistics
National Death Index
National Health Interview Survey
Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392(10159):1923–94.
Barbería-Latasa M, Gea A, Martínez-González MA. Alcohol, Drinking Pattern, and Chronic Disease. Nutrients. 2022;14(9):1954.
Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–45.
Article PubMed Google Scholar
Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342: d671.
Article PubMed PubMed Central Google Scholar
Bergmann MM, Rehm J, Klipstein-Grobusch K, Boeing H, Schütze M, Drogan D, et al. The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int J Epidemiol. 2013;42(6):1772–90.
Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T. Do, “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs. 2016;77(2):185–98.
Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ. 2015;350: h384.
Ferrari P, Licaj I, Muller DC, Kragh Andersen P, Johansson M, Boeing H, et al. Lifetime alcohol use and overall and cause-specific mortality in the European Prospective Investigation into Cancer and nutrition (EPIC) study. BMJ Open. 2014;4(7): e005245.
Bobak M, Malyutina S, Horvat P, Pajak A, Tamosiunas A, Kubinova R, et al. Alcohol, drinking pattern and all-cause, cardiovascular and alcohol-related mortality in Eastern Europe. Eur J Epidemiol. 2016;31(1):21–30.
Ding C, O’Neill D, Bell S, Stamatakis E, Britton A. Association of alcohol consumption with morbidity and mortality in patients with cardiovascular disease: original data and meta-analysis of 48,423 men and women. BMC Med. 2021;19(1):167.
Rehm J, Rovira P, Llamosas-Falcón L, Shield KD. Dose-Response Relationships between Levels of Alcohol Use and Risks of Mortality or Disease, for All People, by Age, Sex, and Specific Risk Factors. Nutrients. 2021;13(8):2652.
Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol. 2010; 55(13):1328–35.
Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J Am Coll Cardiol. 2017; 70(8):913–22.
Ma H, Li X, Zhou T, Sun D, Shai I, Heianza Y, et al. Alcohol consumption levels as compared with drinking habits in predicting all-cause mortality and cause-specific mortality in current drinkers. Mayo Clin Proc. 2021;96(7):1758–69.
Chikritzhs T, Livingston M. Alcohol and the Risk of Injury. Nutrients. 2021;13(8):2777.
Goodman RA, Istre GR, Jordan FB, Herndon JL, Kelaghan J. Alcohol and fatal injuries in Oklahoma. J Stud Alcohol. 1991;52(2):156–61.
Article CAS PubMed Google Scholar
Ker K, Ivers R. Alcohol related harm. Inj Prev. 2006;12(4):273–4.
Article CAS PubMed PubMed Central Google Scholar
Vingilis E, McLeod AI, Stoduto G, Seeley J, Mann RE. Impact of extended drinking hours in Ontario on motor-vehicle collision and non-motor-vehicle collision injuries. J Stud Alcohol Drugs. 2007;68(6):905–11.
Watt K, Purdie DM, Roche AM, McClure RJ. Risk of injury from acute alcohol consumption and the influence of confounders. Addiction. 2004;99(10):1262–73.
Borges G, Cherpitel C, Orozco R, Bond J, Ye Y, Macdonald S, et al. Multicentre study of acute alcohol use and non-fatal injuries: data from the WHO collaborative study on alcohol and injuries. Bull World Health Organ. 2006;84(6):453–60.
Vinson DC, Maclure M, Reidinger C, Smith GS. A population-based case-crossover and case-control study of alcohol and the risk of injury. J Stud Alcohol. 2003;64(3):358–66.
Peck RC, Gebers MA, Voas RB, Romano E. The relationship between blood alcohol concentration (BAC), age, and crash risk. J Safety Res. 2008;39(3):311–9.
Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011;60(1):74–9.
Marques-Vidal P, Vollenweider P, Waeber G. Alcohol consumption and incidence of type 2 diabetes. Results from the CoLaus study. Nutr Metab Cardiovasc Dis. 2015; 25(1):75–84.
Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, et al. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123–32.
Chikritzhs T, Fillmore K, Stockwell T. A healthy dose of scepticism: four good reasons to think again about protective effects of alcohol on coronary heart disease. Drug Alcohol Rev. 2009;28(4):441–4.
Yang L, Zhou M, Sherliker P, Cai Y, Peto R, Wang L, et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int J Epidemiol. 2012;41(4):1101–13.
Lieberoth S, Backer V, Kyvik KO, Skadhauge LR, Tolstrup JS, Grønbæk M, et al. Intake of alcohol and risk of adult-onset asthma. Respir Med. 2012;106(2):184–8.
Ormstad H, Rosness TA, Bergem AL, Bjertness E, Strand BH. Alcohol consumption in the elderly and risk of dementia related death–a Norwegian prospective study with a 17-year follow-up. Int J Neurosci. 2016;126(2):135–44.
Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17(7):542–55.
Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37(5):505–12.
Koning SH, Gansevoort RT, Mukamal KJ, Rimm EB, Bakker SJ, Joosten MM. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int. 2015;87(5):1009–16.
Reynolds K, Gu D, Chen J, Tang X, Yau CL, Yu L, et al. Alcohol consumption and the risk of end-stage renal disease among Chinese men. Kidney Int. 2008;73(7):870–6.
Schaeffner ES, Kurth T, de Jong PE, Glynn RJ, Buring JE, Gaziano JM. Alcohol consumption and the risk of renal dysfunction in apparently healthy men. Arch Intern Med. 2005;165(9):1048–53.
Noble EE, Olson CA, Davis E, Tsan L, Chen YW, Schade R, et al. Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl Psychiatry. 2021;11(1):194.
Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nhis/indexhtm . Accessed 31 Mar 2022.
Schoenborn CA AP. Alcohol use among adults: United States, 1997–98. Advance data from vital and health statistics; no 324 Hyattsville, Maryland: National Center for Health Statistics. 2001.
Li J, Li Y, Ivey KL, Wang DD, Wilkinson JE, Franke A, et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut. 2022;71(4):724–33.
Mirel LB, El Bural FS, Zhang C, Golden C, Cox CS. Comparative analysis of the National Health Interview Survey public-use and restricted-use linked mortality files. Natl Health Stat Report. 2020;143:1–32.
Google Scholar
National Center for Health Statistics. The Linkage of National Center for Health Statistics Survey Data to the National Death Index — 2019 Linked Mortality File (LMF): Linkage Methodology and Analytic Considerations. Hyattsville. 2022.
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34.
Dupont WD, Plummer D. Using Stata v9 to model complex non-linear relationships with restricted cubic splines. In: North American Stata Users' Group Meetings 2005. Stata Users Group. 2005.
Herndon JE 2nd, Harrell FE Jr. The restricted cubic spline as baseline hazard in the proportional hazards model with step function time-dependent covariables. Stat Med. 1995;14(19):2119–29.
Rota M, Bellocco R, Scotti L, Tramacere I, Jenab M, Corrao G, et al. Random-effects meta-regression models for studying nonlinear dose-response relationship, with an application to alcohol and esophageal squamous cell carcinoma. Stat Med. 2010;29(26):2679–87.
SAS Institute Inc. SAS/STAT Software. MI Procedure. Available at: https://support.sas.com/rnd/app/stat/procedures/mi.html . Accessed 2 May 2017.
Jayasekara H, English DR, Room R, MacInnis RJ. Alcohol consumption over time and risk of death: a systematic review and meta-analysis. Am J Epidemiol. 2014;179(9):1049–59.
Choi YJ, Myung SK, Lee JH. Light alcohol drinking and risk of cancer: a meta-analysis of cohort studies. Cancer Res Treat. 2018;50(2):474–87.
Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. 2013;24(2):301–8.
Reilly KH, Gu D, Duan X, Wu X, Chen CS, Huang J, et al. Risk factors for chronic obstructive pulmonary disease mortality in Chinese adults. Am J Epidemiol. 2008;167(8):998–1004.
Millwood IY, Walters RG, Mei XW, Guo Y, Yang L, Bian Z, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. 2019;393(10183):1831–42.
Doll R, Peto R, Hall E, Wheatley K, Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309(6959):911–8.
Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to alcohol consumption: a prospective study among male British doctors. Int J Epidemiol. 2005;34(1):199–204.
Shen C, Ni MY, Schooling CM, Chan WM, Lee SY, Lam TH. Alcohol use and death from respiratory disease in a prospective Chinese elderly cohort study in Hong Kong. Prev Med. 2013;57(6):819–23.
Tabak C, Smit HA, Räsänen L, Fidanza F, Menotti A, Nissinen A, et al. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001;12(2):239–45.
Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307.
Zeisser C, Stockwell TR, Chikritzhs T, Cherpitel C, Ye Y, Gardner C. A systematic review and meta-analysis of alcohol consumption and injury risk as a function of study design and recall period. Alcohol Clin Exp Res. 2013;37 Suppl 1(Suppl 1):E1–8.
Ye Y, Shield K, Cherpitel CJ, Manthey J, Korcha R, Rehm J. Estimating alcohol-attributable fractions for injuries based on data from emergency department and observational studies: a comparison of two methods. Addiction. 2019;114(3):462–70.
Taylor B, Irving HM, Kanteres F, Room R, Borges G, Cherpitel C, et al. The more you drink, the harder you fall: a systematic review and meta-analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend. 2010;110(1–2):108–16.
Andrews SJ, Goate A, Anstey KJ. Association between alcohol consumption and Alzheimer’s disease: a Mendelian randomization study. Alzheimers Dement. 2020;16(2):345–53.
Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care. 2015;38(9):1804–12.
Joosten MM, Grobbee DE, van der AD, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am J Clin Nutr. 2010; 91(6):1777–83.
Berry C, Salim A, Alban R, Mirocha J, Margulies DR, Ley EJ. Serum ethanol levels in patients with moderate to severe traumatic brain injury influence outcomes: a surprising finding. Am Surg. 2010;76(10):1067–70.
Hadjibashi AA, Berry C, Ley EJ, Bukur M, Mirocha J, Stolpner D, et al. Alcohol is associated with a lower pneumonia rate after traumatic brain injury. J Surg Res. 2012;173(2):212–5.
Simou E, Britton J, Leonardi-Bee J. Alcohol and the risk of pneumonia: a systematic review and meta-analysis. BMJ Open. 2018;8(8): e022344.
Buja A, Scafato E, Baggio B, Sergi G, Maggi S, Rausa G, et al. Renal impairment and moderate alcohol consumption in the elderly. Results from the Italian Longitudinal Study on Aging (ILSA). Public Health Nutr. 2011;14(11):1907–18.
Peters H, Martini S, Woydt R, Rückert M, Shimizu F, Kawachi H, et al. Moderate alcohol intake has no impact on acute and chronic progressive anti-thy1 glomerulonephritis. Am J Physiol Renal Physiol. 2003;284(5):F1105–14.
Joo YS, Koh H, Nam KH, Lee S, Kim J, Lee C, et al. Alcohol consumption and progression of chronic kidney disease: results from the Korean cohort study for outcome in patients with chronic kidney disease. Mayo Clin Proc. 2020;95(2):293–305.
White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transpl. 2009;24(8):2464–72.
Article Google Scholar
Download references
Acknowledgements
The authors thank the NCHS of the Centers for Disease Control and Prevention for sharing the NHIS data.
This study is supported by funding from the National Natural Science Foundation of China (NSFC 81973065). The funding bodies had no role in the design of this study, the collection, analysis, and interpretation of the data, or in writing the manuscript.
Author information
Authors and affiliations.
Department of Maternal and Child Health, School of Public Health, Cheeloo College of Medicine, Shandong University, 44 Wenhuaxi Road, Jinan, 250012, China
Yalan Tian, Jiahui Liu, Yue Zhao, Nana Jiang, Xiao Liu & Xia Wang
Department of Cardiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the conception and design of the study and critically revised the manuscript. YT, NJ, and XL were mainly responsible for the data collection. XW, JL, YT, NJ, and XL contributed to the statistical analysis and interpretation of the data. XW, JL, YZ, and GZ assisted in drafting the manuscript. All authors read and approved the final manuscript.

Corresponding author
Correspondence to Xia Wang .
Ethics declarations
Ethics approval and consent to participate.
The National Health Interview Survey data are de-identified and do not include any protected health information. The data are publicly available and exempt under the ethical board review of the corresponding author’s institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: .
Supplementary Materials. ICD-10 codes for causesof death used in this study. Flow chart of the selection of study participants. The distribution of alcohol consumption accordingto NHIS year among NHIS participants in 1997 to 2014.Dose-response relationship between alcohol consumption and risk ofmortality from CVD, chronic lower respiratory tract diseases, accidents, Alzheimer’s disease, diabetes mellitus, influenzaand pneumonia, and nephritis, nephrotic syndrome, or nephrosis mortality. All-cause and cause-specific mortality according to alcoholconsumption status after multiple imputations for variables with missingvalues among NHIS participants in 1997to 2014. All-cause andcause-specific mortalityaccording to alcohol consumption status among NHIS participants in 1997to 2014. All-cause mortality according to alcohol consumptionstatus and NHIS year among NHIS participants in 1997 to2014.Hazards ratios for all-cause and cause-specific mortality according to alcoholconsumption status among NHIS participants in 1997 to 2014. Hazards ratios forall-cause and cause-specific mortality according to alcohol consumptionstatus stratified for sex, age, race/ethnicity and smoking status amongNHIS participants in 1997 to 2014.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tian, Y., Liu, J., Zhao, Y. et al. Alcohol consumption and all-cause and cause-specific mortality among US adults: prospective cohort study. BMC Med 21 , 208 (2023). https://doi.org/10.1186/s12916-023-02907-6
Download citation
Received : 17 January 2023
Accepted : 24 May 2023
Published : 07 June 2023
DOI : https://doi.org/10.1186/s12916-023-02907-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- All-cause mortality
- Cause-specific mortality
- Chronic lower respiratory tract diseases
- Accidents (unintentional injuries)
- Alzheimer’s disease
- Diabetes mellitus
- Influenza and pneumonia
- Nephrotic syndrome
- Or nephrosis
BMC Medicine
ISSN: 1741-7015
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2024
A burden of proof study on alcohol consumption and ischemic heart disease
- Sinclair Carr ORCID: orcid.org/0000-0003-0421-3145 1 ,
- Dana Bryazka 1 ,
- Susan A. McLaughlin 1 ,
- Peng Zheng 1 , 2 ,
- Sarasvati Bahadursingh 3 ,
- Aleksandr Y. Aravkin 1 , 2 , 4 ,
- Simon I. Hay ORCID: orcid.org/0000-0002-0611-7272 1 , 2 ,
- Hilary R. Lawlor 1 ,
- Erin C. Mullany 1 ,
- Christopher J. L. Murray ORCID: orcid.org/0000-0002-4930-9450 1 , 2 ,
- Sneha I. Nicholson 1 ,
- Jürgen Rehm 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ,
- Gregory A. Roth 1 , 2 , 13 ,
- Reed J. D. Sorensen 1 ,
- Sarah Lewington 3 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1 , 2
Nature Communications volume 15 , Article number: 4082 ( 2024 ) Cite this article
6483 Accesses
34 Altmetric
Metrics details
- Cardiovascular diseases
- Epidemiology
- Risk factors
Cohort and case-control data have suggested an association between low to moderate alcohol consumption and decreased risk of ischemic heart disease (IHD), yet results from Mendelian randomization (MR) studies designed to reduce bias have shown either no or a harmful association. Here we conducted an updated systematic review and re-evaluated existing cohort, case-control, and MR data using the burden of proof meta-analytical framework. Cohort and case-control data show low to moderate alcohol consumption is associated with decreased IHD risk – specifically, intake is inversely related to IHD and myocardial infarction morbidity in both sexes and IHD mortality in males – while pooled MR data show no association, confirming that self-reported versus genetically predicted alcohol use data yield conflicting findings about the alcohol-IHD relationship. Our results highlight the need to advance MR methodologies and emulate randomized trials using large observational databases to obtain more definitive answers to this critical public health question.
Similar content being viewed by others

Alcohol consumption and risks of more than 200 diseases in Chinese men
Alcohol intake and the risk of chronic kidney disease: results from a systematic review and dose–response meta-analysis

Association of change in alcohol consumption with cardiovascular disease and mortality among initial nondrinkers
Introduction.
It is well known that alcohol consumption increases the risk of morbidity and mortality due to many health conditions 1 , 2 , with even low levels of consumption increasing the risk for some cancers 3 , 4 . In contrast, a large body of research has suggested that low to moderate alcohol intake – compared to no consumption – is associated with a decreased risk of ischemic heart disease (IHD). This has led to substantial epidemiologic and public health interest in the alcohol-IHD relationship 5 , particularly given the high prevalence of alcohol consumption 6 and the global burden of IHD 7 .
Extensive evidence from experimental studies that vary short-term alcohol exposure suggests that average levels of alcohol intake positively affect biomarkers such as apolipoprotein A1, adiponectin, and fibrinogen levels that lower the risk of IHD 8 . In contrast, heavy episodic drinking (HED) may have an adverse effect on IHD by affecting blood lipids, promoting coagulation and thus thrombosis risk, and increasing blood pressure 9 . With effects likely to vary materially by patterns of drinking, alcohol consumption must be considered a multidimensional factor impacting IHD outcomes.
A recent meta-analysis of the alcohol-IHD relationship using individual participant data from 83 observational studies 4 found, among current drinkers, that – relative to drinking less than 50 g/week – any consumption above this level was associated with a lower risk of myocardial infarction (MI) incidence and consumption between >50 and <100 g/week was associated with lower risk of MI mortality. When evaluating other subtypes of IHD excluding MI, the researchers found that consumption between >100 and <250 g/week was associated with a decreased risk of IHD incidence, whereas consumption greater than 350 g/week was associated with an increased risk of IHD mortality. Roerecke and Rehm further observed that low to moderate drinking was not associated with reduced IHD risk when accompanied by occasional HED 10 .
The cohort studies and case-control studies (hereafter referred to as ‘conventional observational studies’) used in these meta-analyses are known to be subject to various types of bias when used to estimate causal relationships 11 . First, neglecting to separate lifetime abstainers from former drinkers, some of whom may have quit due to developing preclinical symptoms (sometimes labeled ‘sick quitters’ 12 , 13 ), and to account for drinkers who reduce their intake as a result of such symptoms may introduce reverse causation bias 13 . That is, the risk of IHD in, for example, individuals with low to moderate alcohol consumption may be lower when compared to IHD risk in sick quitters, not necessarily because intake at this level causes a reduction in risk but because sick quitters are at higher risk of IHD. Second, estimates can be biased because of measurement error in alcohol exposure resulting from inaccurate reporting, random fluctuation in consumption over time (random error), or intentional misreporting of consumption due, for example, to social desirability effects 14 (systematic error). Third, residual confounding may bias estimates if confounders of the alcohol-IHD relationship, such as diet or physical activity, have not been measured accurately (e.g., only via a self-report questionnaire) or accounted for. Fourth, because alcohol intake is a time-varying exposure, time-varying confounding affected by prior exposure must be accounted for 15 . To date, only one study that used a marginal structural model to appropriately adjust for time-varying confounding found no association between alcohol consumption and MI risk 16 . Lastly, if exposure to a risk factor, such as alcohol consumption, did not happen at random – even if all known confounders of the relationship between alcohol and IHD were perfectly measured and accounted for – the potential for unmeasured confounders persists and may bias estimates 11 .
In recent years, the analytic method of Mendelian randomization (MR) has been widely adopted to quantify the causal effects of risk factors on health outcomes 17 , 18 , 19 . MR uses single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) for the exposure of interest. A valid IV should fulfill the following three assumptions: it must be associated with the risk factor (relevance assumption); there must be no common causes of the IV and the outcome (independence assumption); and the IV must affect the outcome only through the exposure (exclusion restriction or ‘no horizontal pleiotropy’ assumption) 20 , 21 . If all three assumptions are fulfilled, estimates derived from MR are presumed to represent causal effects 22 . Several MR studies have quantified the association between alcohol consumption and cardiovascular disease 23 , including IHD, using genes known to impact alcohol metabolism (e.g., ADH1B/C and ALDH2 24 ) or SNP combinations from genome-wide association studies 25 . In contrast to the inverse associations found in conventional observational studies, MR studies have found either no association or a harmful relationship between alcohol consumption and IHD 26 , 27 , 28 , 29 , 30 , 31 .
To advance the knowledge base underlying our understanding of this major health issue – critical given the worldwide ubiquity of alcohol use and of IHD – there is a need to systematically review and critically re-evaluate all available evidence on the relationship between alcohol consumption and IHD risk from both conventional observational and MR studies.
The burden of proof approach, developed by Zheng et al. 32 , is a six-step meta-analysis framework that provides conservative estimates and interpretations of risk-outcome relationships. The approach systematically tests and adjusts for common sources of bias defined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria: representativeness of the study population, exposure assessment, outcome ascertainment, reverse causation, control for confounding, and selection bias. The key statistical tool to implement the approach is MR-BRT (meta-regression—Bayesian, regularized, trimmed 33 ), a flexible meta-regression tool that does not impose a log-linear relationship between the risk and outcome, but instead uses a spline ensemble to model non-linear relationships. MR-BRT also algorithmically detects and trims outliers in the input data, takes into account different reference and alternative exposure intervals in the data, and incorporates unexplained between-study heterogeneity in the uncertainty surrounding the mean relative risk (RR) curve (henceforth ‘risk curve’). For those risk-outcome relationships that meet the condition of statistical significance using conventionally estimated uncertainty intervals (i.e., without incorporating unexplained between-study heterogeneity), the burden of proof risk function (BPRF) is derived by calculating the 5th (if harmful) or 95th (if protective) quantile risk curve – inclusive of between-study heterogeneity – closest to the log RR of 0. The resulting BPRF is a conservative interpretation of the risk-outcome relationship based on all available evidence. The BPRF represents the smallest level of excess risk for a harmful risk factor or reduced risk for a protective risk factor that is consistent with the data, accounting for between-study heterogeneity. To quantify the strength of the evidence for the alcohol-IHD relationship, the BPRF can be summarized in a single metric, the risk-outcome score (ROS). The ROS is defined as the signed value of the average log RR of the BPRF across the 15th to 85th percentiles of alcohol consumption levels observed across available studies. The larger a positive ROS value, the stronger the alcohol-IHD association. For ease of interpretation, the ROS is converted into a star rating from one to five. A one-star rating (ROS < 0) indicates a weak alcohol-IHD relationship, and a five-star rating (ROS > 0.62) indicates a large effect size and strong evidence. Publication and reporting bias are evaluated with Egger’s regression and by visual inspection with funnel plots 34 . Further conceptual and technical details of the burden of proof approach are described in detail elsewhere 32 .
Using the burden of proof approach, we systematically re-evaluate all available eligible evidence from cohort, case-control, and MR studies published between 1970 and 2021 to conservatively quantify the dose-response relationship between alcohol consumption and IHD risk, calculated relative to risk at zero alcohol intake (i.e., current non-drinking, including lifetime abstinence or former use). We pool the evidence from all conventional observational studies combined, as well as individually for all three study designs, to estimate mean IHD risk curves. Based on patterns of results established by previous meta-analyses 4 , 35 , we also use data from conventional observational studies to estimate risk curves by IHD endpoint (morbidity or mortality) and further by sex, in addition to estimating risk curves for MI overall and by endpoint. We follow PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines 36 through all stages of this study (Supplementary Information section 1 , Fig. S1 and Tables S1 and S2 ) and comply with GATHER (Guidelines on Accurate and Transparent Health Estimates Reporting) recommendations 37 (Supplementary Information section 2 , Table S3 ). The main findings and research implications of this work are summarized in Table 1 .
We updated the systematic review on the dose-response relationship between alcohol consumption and IHD previously conducted for the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 1 . Of 4826 records identified in our updated systematic review (4769 from databases/registers and 57 by citation search and known literature), 11 were eligible based on our inclusion criteria and were included. In total, combined with the results of the previous systematic reviews 1 , 38 , information from 95 cohort studies 26 , 27 , 29 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 27 case-control studies 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , and five MR studies 26 , 27 , 28 , 29 , 31 was included in our meta-analysis (see Supplementary Information section 1 , Fig. S1 , for the PRISMA diagram). Details on the extracted effect sizes, the design of each included study, underlying data sources, number of participants, duration of follow-up, number of cases and controls, and bias covariates that were evaluated and potentially adjusted for can be found in the Supplementary Information Sections 4 , 5 , and 6 .
Table 2 summarizes key metrics of each risk curve modeled, including estimates of mean RR and 95% UI (inclusive of between-study heterogeneity) at select alcohol exposure levels, the exposure level and RR and 95% UI at the nadir (i.e., lowest RR), the 85th percentile of exposure observed in the data and its corresponding RR and 95% UI, the BPRF averaged at the 15th and 85th percentile of exposure, the average excess risk or risk reduction according to the exposure-averaged BPRF, the ROS, the associated star rating, the potential presence of publication or reporting bias, and the number of studies included.
We found large variation in the association between alcohol consumption and IHD by study design. When we pooled the results of cohort and case-control studies, we observed an inverse association between alcohol at average consumption levels and IHD risk; that is, drinking average levels of alcohol was associated with a reduced IHD risk relative to drinking no alcohol. In contrast, we did not find a statistically significant association between alcohol consumption and IHD risk when pooling results from MR studies. When we subset the conventional observational studies to those reporting on IHD by endpoint, we found no association between alcohol consumption and IHD morbidity or mortality due to large unexplained heterogeneity between studies. When we further subset those studies that reported effect size estimates by sex, we found that average alcohol consumption levels were inversely associated with IHD morbidity in males and in females, and with IHD mortality in males but not in females. When we analyzed only the studies that reported on MI, we found significant inverse associations between average consumption levels and MI overall and with MI morbidity. Visualizations of the risk curves for morbidity and mortality of IHD and MI are provided in Supplementary Information Section 9 (Figs. S2a –c, S3a –c, and S4a–c ). Among all modeled risk curves for which a BPRF was calculated, the ROS ranged from −0.40 for MI mortality to 0.20 for MI morbidity. In the Supplementary Information, we also provide details on the RR and 95% UIs with and without between-study heterogeneity associated with each 10 g/day increase in consumption for each risk curve (Table S10 ), the parameter specifications of the model (Tables S11 and S12 ), and each risk curve from the main analysis estimated without trimming 10% of the data (Fig. S5a–l and Table S13 ).
Risk curve derived from conventional observational study data
The mean risk curve and 95% UI were first estimated by combining all evidence from eligible cohort and case-control studies that quantified the association between alcohol consumption and IHD risk. In total, information from 95 cohort studies and 27 case-control studies combining data from 7,059,652 participants were included. In total, 243,357 IHD events were recorded. Thirty-seven studies quantified the association between alcohol consumption and IHD morbidity only, and 44 studies evaluated only IHD mortality. The estimated alcohol-IHD association was adjusted for sex and age in all but one study. Seventy-five studies adjusted the effect sizes for sex, age, smoking, and at least four other covariates. We adjusted our risk curve for whether the study sample was under or over 50 years of age, whether the study outcome was consistent with the definition of IHD (according to the International Classification of Diseases [ICD]−9: 410-414; and ICD-10: I20-I25) or related to specified subtypes of IHD, whether the outcome was ascertained by self-report only or by at least one other measurement method, whether the study accounted for risk for reverse causation, whether the reference group was non-drinkers (including lifetime abstainers and former drinkers), and whether effect sizes were adjusted (1) for sex, age, smoking, and at least four other variables, (2) for apolipoprotein A1, and (3) for cholesterol, as these bias covariates were identified as significant by our algorithm.
Pooling all data from cohort and case-control studies, we found that alcohol consumption was inversely associated with IHD risk (Fig. 1 ). The risk curve was J-shaped – without crossing the null RR of 1 at high exposure levels – with a nadir of 0.69 (95% UI: 0.48–1.01) at 23 g/day. This means that compared to individuals who do not drink alcohol, the risk of IHD significantly decreases with increasing consumption up to 23 g/day, followed by a risk reduction that becomes less pronounced. The average BPRF calculated between 0 and 45 g/day of alcohol intake (the 15th and 85th percentiles of the exposure range observed in the data) was 0.96. Thus, when between-study heterogeneity is accounted for, a conservative interpretation of the evidence suggests drinking alcohol across the average intake range is associated with an average decrease in the risk of IHD of at least 4% compared to drinking no alcohol. This corresponds to a ROS of 0.04 and a star rating of two, which suggests that the association – on the basis of the available evidence – is weak. Although we algorithmically identified and trimmed 10% of the data to remove outliers, Egger’s regression and visual inspection of the funnel plot still indicated potential publication or reporting bias.
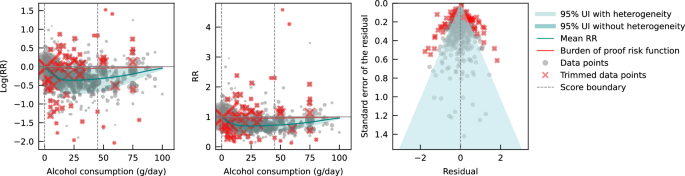
The panels show the log(relative risk) function, the relative risk function, and a modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard error that includes the reported standard error and between-study heterogeneity on the y-axis. RR relative risk, UI uncertainty interval. Source data are provided as a Source Data file.
Risk curve derived from case-control study data
Next, we estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD by subsetting the data to case-control studies only. We included a total of 27 case-control studies (including one nested case-control study) with data from 60,914 participants involving 16,892 IHD cases from Europe ( n = 15), North America ( n = 6), Asia ( n = 4), and Oceania ( n = 2). Effect sizes were adjusted for sex and age in most studies ( n = 25). Seventeen of these studies further adjusted for smoking and at least four other covariates. The majority of case-control studies accounted for the risk of reverse causation ( n = 25). We did not adjust our risk curve for bias covariates, as our algorithm did not identify any as significant.
Evaluating only data from case-control studies, we observed a J-shaped relationship between alcohol consumption and IHD risk, with a nadir of 0.65 (0.50–0.85) at 23 g/day (Fig. 2 ). The inverse association between alcohol consumption and IHD risk reversed at an intake level of 61 g/day. In other words, alcohol consumption between >0 and 60 g/day was associated with a lower risk compared to no consumption, while consumption at higher levels was associated with increased IHD risk. However, the curve above this level is flat, implying that the association between alcohol and increased IHD risk is the same between 61 and 100 g/day, relative to not drinking any alcohol. The BPRF averaged across the exposure range between the 15th and 85th percentiles, or 0–45 g/day, was 0.87, which translates to a 13% average reduction in IHD risk across the average range of consumption. This corresponds to a ROS of 0.14 and a three-star rating. After trimming 10% of the data, no potential publication or reporting bias was found.
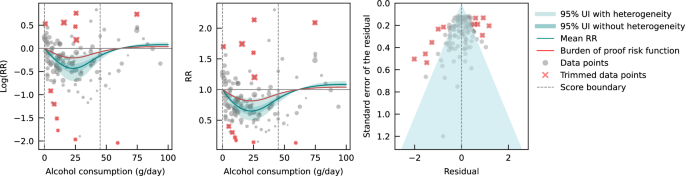
The panels show the log(relative risk) function, the relative risk function, and a modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard deviation that includes the reported standard deviation and between-study heterogeneity on the y-axis. RR relative risk, UI uncertainty interval. Source data are provided as a Source Data file.
Risk curve derived from cohort study data
We also estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD using only data from cohort studies. In total, 95 cohort studies – of which one was a retrospective cohort study – with data from 6,998,738 participants were included. Overall, 226,465 IHD events were recorded. Most data were from Europe ( n = 43) and North America ( n = 33), while a small number of studies were conducted in Asia ( n = 14), Oceania ( n = 3), and South America ( n = 2). The majority of studies adjusted effect sizes for sex and age ( n = 76). Fifty-seven of these studies also adjusted for smoking and at least four other covariates. Out of all cohort studies included, 88 accounted for the risk of reverse causation. We adjusted our risk curve for whether the study outcome was consistent with the definition of IHD or related to specified subtypes of IHD, and whether effect sizes were adjusted for apolipoprotein A1, as these bias covariates were identified as significant by our algorithm.
When only data from cohort studies were evaluated, we found a J-shaped relationship between alcohol consumption and IHD risk that did not cross the null RR of 1 at high exposure levels, with a nadir of 0.69 (0.47–1.01) at 23 g/day (Fig. 3 ). The shape of the risk curve was almost identical to the curve estimated with all conventional observational studies (i.e., cohort and case-control studies combined). When we calculated the average BPRF of 0.95 between the 15th and 85th percentiles of observed alcohol exposure (0–50 g/day), we found that alcohol consumption across the average intake range was associated with an average reduction in IHD risk of at least 5%. This corresponds to a ROS of 0.05 and a two-star rating. We identified potential publication or reporting bias after 10% of the data were trimmed.
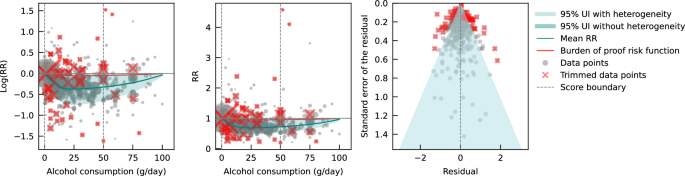
Risk curve derived from Mendelian randomization study data
Lastly, we pooled evidence on the relationship between genetically predicted alcohol consumption and IHD risk from MR studies. Four MR studies were considered eligible for inclusion in our main analysis, with data from 559,708 participants from China ( n = 2), the Republic of Korea ( n = 1), and the United Kingdom ( n = 1). Overall, 22,134 IHD events were recorded. Three studies used the rs671 ALDH2 genotype found in Asian populations, one study additionally used the rs1229984 ADH1B variant, and one study used the rs1229984 ADH1B Arg47His variant and a combination of 25 SNPs as IVs. All studies used the two-stage least squares (2SLS) method to estimate the association, and one study additionally applied the inverse-variance-weighted (IVW) method and multivariable MR (MVMR). For the study that used multiple methods to estimate effect sizes, we used the 2SLS estimates for our main analysis. Further details on the included studies are provided in Supplementary Information section 4 (Table S6 ). Due to limited input data, we elected not to trim 10% of the observations. We adjusted our risk curve for whether the endpoint of the study outcome was mortality and whether the associations were adjusted for sex and/or age, as these bias covariates were identified as significant by our algorithm.
We did not find any significant association between genetically predicted alcohol consumption and IHD risk using data from MR studies (Fig. 4 ). No potential publication or reporting bias was detected.
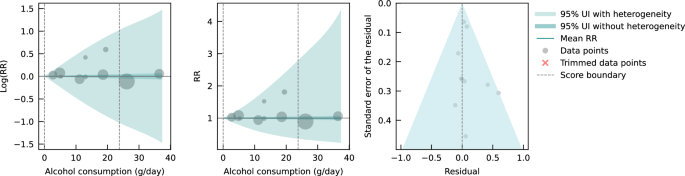
As sensitivity analyses, we modeled risk curves with effect sizes estimated from data generated by Lankester et al. 28 using IVW and MVMR methods. We also used effect sizes from Biddinger et al. 31 , obtained using non-linear MR with the residual method, instead of those from Lankester et al. 28 in our main model (both were estimated with UK Biobank data) to estimate a risk curve. Again, we did not find a significant association between genetically predicted alcohol consumption and IHD risk (see Supplementary Information Section 10 , Fig. S6a–c and Table S14 ). To test for consistency with the risk curve we estimated using all included cohort studies, we also pooled the conventionally estimated effect sizes provided in the four MR studies. We did not observe an association between alcohol consumption and IHD risk due to large unexplained heterogeneity between studies (see Supplementary Information Section 10 , Fig. S7, and Table S14 ). Lastly, we pooled cohort studies that included data from China, the Republic of Korea, and the United Kingdom to account for potential geographic influences. Again, we did not find a significant association between alcohol consumption and IHD risk (see Supplementary Information Section 10 , Fig. S8, and Table S14 ).
Conventional observational and MR studies published to date provide conflicting estimates of the relationship between alcohol consumption and IHD. We conducted an updated systematic review and conservatively re-evaluated existing evidence on the alcohol-IHD relationship using the burden of proof approach. We synthesized evidence from cohort and case-control studies combined and separately and from MR studies to assess the dose-response relationship between alcohol consumption and IHD risk and to compare results across different study designs. It is anticipated that the present synthesis of evidence will be incorporated into upcoming iterations of GBD.
Our estimate of the association between genetically predicted alcohol consumption and IHD runs counter to our estimates from the self-report data and those of other previous meta-analyses 4 , 35 , 158 that pooled conventional observational studies. Based on the conservative burden of proof interpretation of the data, our results suggested an inverse association between alcohol and IHD when all conventional observational studies were pooled (alcohol intake was associated with a reduction in IHD risk by an average of at least 4% across average consumption levels; two-star rating). In evaluating only cohort studies, we again found an inverse association between alcohol consumption and IHD (alcohol intake was associated with a reduction in IHD risk by an average of at least 5% at average consumption levels; two-star rating). In contrast, when we pooled only case-control studies, we estimated that average levels of alcohol consumption were associated with at least a 13% average decrease in IHD risk (three-star rating), but the inverse association reversed when consumption exceeded 60 g/day, suggesting that alcohol above this level is associated with a slight increase in IHD risk. Our analysis of the available evidence from MR studies showed no association between genetically predicted alcohol consumption and IHD.
Various potential biases and differences in study designs may have contributed to the conflicting findings. In our introduction, we summarized important sources of bias in conventional observational studies of the association between alcohol consumption and IHD. Of greatest concern are residual and unmeasured confounding and reverse causation, the effects of which are difficult to eliminate in conventional observational studies. By using SNPs within an IV approach to predict exposure, MR – in theory – eliminates these sources of bias and allows for more robust estimates of causal effects. Bias may still occur, however, when using MR to estimate the association between alcohol and IHD 159 , 160 . There is always the risk of horizontal pleiotropy in MR – that is, the genetic variant may affect the outcome via pathways other than exposure 161 . The IV assumption of exclusion restriction is, for example, violated if only a single measurement of alcohol consumption is used in MR 162 ; because alcohol consumption varies over the life course, the gene directly impacts IHD through intake at time points other than that used in the MR analysis. To date, MR studies have not succeeded in separately capturing the multidimensional effects of alcohol intake on IHD risk (i.e., effects of average alcohol consumption measured through frequency-quantity, in addition to the effects of HED) 159 because the genes used to date only target average alcohol consumption that encompasses intake both at average consumption levels and HED. In other words, the instruments used are not able to separate out the individual effects of these two different dimensions of alcohol consumption on IHD risk using MR. Moreover, reverse causation may occur through cross-generational effects 160 , 163 , as the same genetic variants predispose both the individual and at least one of his or her parents to (increased) alcohol consumption. In this situation, IHD risk could be associated with the parents’ genetically predicted alcohol consumption and not with the individual’s own consumption. None of the MR studies included accounted for cross-generational effects, which possibly introduced bias in the effect estimates. It is important to note that bias by ancestry might also occur in conventional observational studies 164 . In summary, estimates of the alcohol-IHD association are prone to bias in all three study designs, limiting inferences of causation.
The large difference in the number of available MR versus conventional observational studies, the substantially divergent results derived from the different study types, and the rapidly developing field of MR clearly argue for further investigation of MR as a means to quantify the association between alcohol consumption and IHD risk. Future studies should investigate non-linearity in the relationship using non-linear MR methods. The residual method, commonly applied in non-linear MR studies such as Biddinger et al. 31 , assumes a constant, linear relationship between the genetic IV and the exposure in the study population; a strong assumption that may result in biased estimates and inflated type I error rates if the relationship varies by population strata 165 . However, by log-transforming the exposure, the relationships between the genetic IV and the exposure as expressed on a logarithmic scale may be more homogeneous across strata, possibly reducing the bias effect of violating the assumption of a constant, linear relationship. Alternatively, or in conjunction, the recently developed doubly ranked method, which obviates the need for this assumption, could be used 166 . Since methodology for non-linear MR is an active field of study 167 , potential limitations of currently available methods should be acknowledged and latest guidelines be followed 168 . Future MR studies should further (i) employ sensitivity analyses such as the MR weighted median method 169 to relax the exclusion restriction assumption that may be violated, as well as applying other methods such as the MR-Egger intercept test; (ii) use methods such as g-estimation of structural mean models 162 to adequately account for temporal variation in alcohol consumption in MR, and (iii) attempt to disaggregate the effects of alcohol on IHD by dimension in MR, potentially through the use of MVMR 164 . General recommendations to overcome common MR limitations are described in greater detail elsewhere 159 , 163 , 170 , 171 and should be carefully considered. With respect to prospective cohort studies used to assess the alcohol-IHD relationship, they should, at a minimum: (i) adjust the association between alcohol consumption and IHD for all potential confounders identified, for example, using a causal directed acyclic graph, and (ii) account for reverse causation introduced by sick quitters and by drinkers who changed their consumption. If possible, they should also (iii) use alcohol biomarkers as objective measures of alcohol consumption instead of or in addition to self-reported consumption to reduce bias through measurement error, (iv) investigate the association between IHD and HED, in addition to average alcohol consumption, and (v) when multiple measures of alcohol consumption and potential confounders are available over time, use g-methods to reduce bias through confounding as fully as possible within the limitations of the study design. However, some bias – due, for instance, to unmeasured confounding in conventional observational and to horizontal pleiotropy in MR studies – is likely inevitable, and the interpretation of estimates should be appropriately cautious, in accordance with the methods used in the study.
With the introduction of the Moderate Alcohol and Cardiovascular Health Trial (MACH15) 172 , randomized controlled trials (RCTs) have been revisited as a way to study the long-term effects of low to moderate alcohol consumption on cardiovascular disease, including IHD. In 2018, soon after the initiation of MACH15, the National Institutes of Health terminated funding 173 , reportedly due to concerns about study design and irregularities in the development of funding opportunities 174 . Although MACH15 was terminated, its initiation represented a previously rarely considered step toward investigating the alcohol-IHD relationship using an RCT 175 . However, while the insights from an RCT are likely to be invaluable, the implementation is fraught with potential issues. Due to the growing number of studies suggesting increased disease risk, including cancer 3 , 4 , associated with alcohol use even at very low levels 176 , the use of RCTs to study alcohol consumption is ethically questionable 177 . A less charged approach could include the emulation of target trials 178 using existing observational data (e.g., from large-scale prospective cohort studies such as the UK Biobank 179 , Atherosclerosis Risk in Communities Study 180 , or the Framingham Heart Study 181 ) in lieu of real trials to gather evidence on the potential cardiovascular effects of alcohol. Trials like MACH15 can be emulated, following the proposed trial protocols as closely as the observational dataset used for the analysis allows. Safety and ethical concerns, such as those related to eligibility criteria, initiation/increase in consumption, and limited follow-up duration, will be eliminated because the data will have already been collected. This framework allows for hypothetical trials investigating ethically challenging or even untenable questions, such as the long-term effects of heavy (episodic) drinking on IHD risk, to be emulated and inferences to broader populations drawn.
There are several limitations that must be considered when interpreting our findings. First, record screening for our systematic review was not conducted in a double-blinded fashion. Second, we did not have sufficient evidence to estimate and examine potential differential associations of alcohol consumption with IHD risk by beverage type or with MI endpoints by sex. Third, despite using a flexible meta-regression tool that overcame several limitations common to meta-analyses, the results of our meta-analysis were only as good as the quality of the studies included. We were able, however, to address the issue of varying quality of input data by adjusting for bias covariates that corresponded to core study characteristics in our analyses. Fourth, because we were only able to include one-sample MR studies that captured genetically predicted alcohol consumption, statistical power may be lower than would have been possible with the inclusion of two-sample MR studies, and studies that directly estimated gene-IHD associations were not considered 23 . Finally, we were not able to account for participants’ HED status when pooling effect size estimates from conventional observational studies. Given established differences in IHD risk for drinkers with and without HED 35 and the fact that more than one in three drinkers reports HED 6 , we would expect that the decreased average risk we found at moderate levels of alcohol consumption would be attenuated (i.e., approach the IHD risk of non-drinkers) if the presence of HED was taken into account.
Using the burden of proof approach 32 , we conservatively re-evaluated the dose-response relationship between alcohol consumption and IHD risk based on existing cohort, case-control, and MR data. Consistent with previous meta-analyses, we found that alcohol at average consumption levels was inversely associated with IHD when we pooled conventional observational studies. This finding was supported when aggregating: (i) all studies, (ii) only cohort studies, (iii) only case-control studies, (iv) studies examining IHD morbidity in females and males, (v) studies examining IHD mortality in males, and (vi) studies examining MI morbidity. In contrast, we found no association between genetically predicted alcohol consumption and IHD risk based on data from MR studies. Our confirmation of the conflicting results derived from self-reported versus genetically predicted alcohol use data highlights the need to advance methodologies that will provide more definitive answers to this critical public health question. Given the limitations of randomized trials, we advocate using advanced MR techniques and emulating target trials using observational data to generate more conclusive evidence on the long-term effects of alcohol consumption on IHD risk.
This study was approved by the University of Washington IRB Committee (study #9060).
The burden of proof approach is a six-step framework for conducting meta-analysis 32 : (1) data from published studies that quantified the dose-response relationship between alcohol consumption and ischemic heart disease (IHD) risk were systematically identified and obtained; (2) the shape of the mean relative risk (RR) curve (henceforth ‘risk curve’) and associated uncertainty was estimated using a quadratic spline and algorithmic trimming of outliers; (3) the risk curve was tested and adjusted for biases due to study attributes; (4) unexplained between-study heterogeneity was quantified, adjusting for within-study correlation and number of studies included; (5) the evidence for small-study effects was evaluated to identify potential risks of publication or reporting bias; and (6) the burden of proof risk function (BPRF) – a conservative interpretation of the average risk across the exposure range found in the data – was estimated relative to IHD risk at zero alcohol intake. The BPRF was converted to a risk-outcome score (ROS) that was mapped to a star rating from one to five to provide an intuitive interpretation of the magnitude and direction of the dose-response relationship between alcohol consumption and IHD risk.
We calculated the mean RR and 95% uncertainty intervals (UIs) for IHD associated with levels of alcohol consumption separately with all evidence available from conventional observational studies and from Mendelian randomization (MR) studies. For the risk curves that met the condition of statistical significance when the conventional 95% UI that does not include unexplained between-study heterogeneity was evaluated, we calculated the BPRF, ROS, and star rating. Based on input data from conventional observational studies, we also estimated these metrics by study design (cohort studies, case-control studies), and by IHD endpoint (morbidity, mortality) for both sexes (females, males) and sex-specific. For sex-stratified analyses, we only considered studies that reported effect sizes for both females and males to allow direct comparison of IHD risk across different exposure levels; however, we did not collect information about the method each study used to determine sex. We also estimated risk curves for myocardial infarction (MI), overall and by endpoint, using data from conventional observational studies. As a comparison, we also estimated each risk curve without trimming 10% of the input data. We did not consider MI as an outcome or disaggregate findings by sex or endpoint for MR studies due to insufficient data.
With respect to MR studies, several statistical methods are typically used to estimate the associations between genetically predicted exposure and health outcomes (e.g., two-stage least squares [2SLS], inverse-variance-weighted [IVW], multivariable Mendelian randomization [MVMR]). For our main analysis synthesizing evidence from MR studies, we included the reported effect sizes estimated using 2SLS if a study applied multiple methods because this method was common to all included studies. In sensitivity analyses, we used the effect sizes obtained by other MR methods (i.e., IVW, MVMR, and non-linear MR) and estimated the mean risk curve and uncertainty. We also pooled conventionally estimated effect sizes from MR studies to allow comparison with the risk curve estimated with cohort studies. Due to limited input data from MR studies, we elected not to trim 10% of the observations. Furthermore, we estimated the risk curve from cohort studies with data from countries that corresponded to those included in MR studies (China, the Republic of Korea, and the United Kingdom). Due to a lack of data, we were unable to estimate a risk curve from case-control studies in these geographic regions.
Conducting the systematic review
In step one of the burden of proof approach, data for the dose-response relationship between alcohol consumption and IHD risk were systematically identified, reviewed, and extracted. We updated a previously published systematic review 1 in PubMed that identified all studies evaluating the dose-response relationship between alcohol consumption and risk of IHD morbidity or mortality from January 1, 1970, to December 31, 2019. In our update, we additionally considered all studies up to and including December 31, 2021, for eligibility. We searched articles in PubMed on March 21, 2022, with the following search string: (alcoholic beverage[MeSH Terms] OR drinking behavior[MeSH Terms] OR “alcohol”[Title/Abstract]) AND (Coronary Artery Disease[Mesh] OR Myocardial Ischemia[Mesh] OR atherosclerosis[Mesh] OR Coronary Artery Disease[TiAb] OR Myocardial Ischemia[TiAb] OR cardiac ischemia[TiAb] OR silent ischemia[TiAb] OR atherosclerosis Outdent [TiAb] OR Ischemic heart disease[TiAb] OR Ischemic heart disease[TiAb] OR coronary heart disease[TiAb] OR myocardial infarction[TiAb] OR heart attack[TiAb] OR heart infarction[TiAb]) AND (Risk[MeSH Terms] OR Odds Ratio[MeSH Terms] OR “risk”[Title/Abstract] OR “odds ratio”[Title/Abstract] OR “cross-product ratio”[Title/Abstract] OR “hazards ratio”[Title/Abstract] OR “hazard ratio”[Title/Abstract]) AND (“1970/01/01”[PDat]: “2021/12/31”[PDat]) AND (English[LA]) NOT (animals[MeSH Terms] NOT Humans[MeSH Terms]). Studies were eligible for inclusion if they met all of the following criteria: were published between January 1, 1970, and December 31, 2021; were a cohort study, case-control study, or MR study; described an association between alcohol consumption and IHD and reported an effect size estimate (relative risk, hazard ratio, odds ratio); and used a continuous dose as exposure of alcohol consumption. Studies were excluded if they met any of the following criteria: were an aggregate study (meta-analysis or pooled cohort); utilized a study design not designated for inclusion in this analysis: not a cohort study, case-control study, or MR study; were a duplicate study: the underlying sample of the study had also been analyzed elsewhere (we always considered the analysis with the longest follow-up for cohort studies or the most recently published analysis for MR studies); did not report on the exposure of interest: reported on combined exposure of alcohol and drug use or reported alcohol consumption in a non-continuous way; reported an outcome that was not IHD or a composite outcome that included but was not limited to IHD, or outcomes lacked specificity, such as cardiovascular disease or all-cause mortality; were not in English; and were animal studies. All screenings of titles and abstracts of identified records, as well as full texts of potentially eligible studies, and extraction of included studies, were done by a single reviewer (SC or HL) independently. If eligible, studies were extracted for study characteristics, exposure, outcome, adjusted confounders, and effect sizes and their uncertainty. While the previous systematic review only considered cohort and case-control studies, our update also included MR studies. We chose to consider only ‘one-sample’ MR studies, i.e., those in which genes, risk factors, and outcomes were measured in the same participants, and not ‘two-sample’ MR studies in which two different samples were used for the MR analysis so that we could fully capture study-specific information. We re-screened previously identified records for MR studies to consider all published MR studies in the defined time period. We also identified and included in our sensitivity analysis an MR study published in 2022 31 which used a non-linear MR method to estimate the association between genetically predicted alcohol consumption and IHD. When eligible studies reported both MR and conventionally estimated effect sizes (i.e., for the association between self-reported alcohol consumption and IHD risk), we extracted both. If studies used the same underlying sample and investigated the same outcome in the same strata, we included the study that had the longest follow-up. This did not apply when the same samples were used in conventional observational and MR studies, because they were treated separately when estimating the risk curve of alcohol consumption and IHD. Continuous exposure of alcohol consumption was defined as a frequency-quantity measure 182 and converted to g/day. IHD was defined according to the International Classification of Diseases (ICD)−9, 410-414, and ICD-10, I20-I25.
The raw data were extracted with a standardized extraction sheet (see Supplementary Information Section 3 , Table S4 ). For conventional observational studies, when multiple effect sizes were estimated from differently adjusted regression models, we used those estimated with the model reported to be fully adjusted or the one with the most covariates. In the majority of studies, alcohol consumption was categorized based on the exposure range available in the data. If the lower end of a categorical exposure range (e.g., <10 g/day) of an effect size was not specified in the input data, we assumed that this was 0 g/day. If the upper end was not specified (e.g., >20 g/day), it was calculated by multiplying the lower end of the categorical exposure range by 1.5. When the association between alcohol and IHD risk was reported as a linear slope, the average consumption level in the sample was multiplied by the logarithm of the effect size to effectively render it categorical. From the MR study which employed non-linear MR 31 , five effect sizes and their uncertainty were extracted at equal intervals across the reported range of alcohol exposure using WebPlotDigitizer. To account for the fact that these effect sizes were derived from the same non-linear risk curve, we adjusted the extracted standard errors by multiplying them by the square root of five (i.e., the number of extracted effect sizes). Details on data sources are provided in Supplementary Information Section 4 .
Estimating the shape of the risk-outcome relationship
In step two, the shape of the dose-response relationship (i.e., ‘signal’) between alcohol consumption and IHD risk was estimated relative to risk at zero alcohol intake. The meta-regression tool MR-BRT (meta-regression—Bayesian, regularized, trimmed), developed by Zheng et al. 33 , was used for modeling. To allow for non-linearity, thus relaxing the common assumption of a log-linear relationship, a quadratic spline with two interior knots was used for estimating the risk curve 33 . We used the following three risk measures from included studies: RRs, odds ratios (ORs), and hazard ratios (HRs). ORs were treated as equivalent to RRs and HRs based on the rare outcome assumption. To counteract the potential influence of knot placement on the shape of the risk curve when using splines, an ensemble model approach was applied. Fifty component models with random knot placements across the exposure domain were computed. These were combined into an ensemble by weighting each model based on model fit and variation (i.e., smoothness of fit to the data). To prevent bias from outliers, a robust likelihood-based approach was applied to trim 10% of the observations. Technical details on estimating the risk curve, use of splines, the trimming procedure, the ensemble model approach, and uncertainty estimation are described elsewhere 32 , 33 . Details on the model specifications for each risk curve are provided in Supplementary Information section 8 . We first estimated each risk curve without trimming input data to visualize the shape of the curve, which informed knot placement and whether to set a left and/or right linear tail when data were sparse at low or high exposure levels (see Supplementary Information Section 10 , Fig. S5a–l ).
Testing and adjusting for biases across study designs and characteristics
In step three, the risk curve was tested and adjusted for systematic biases due to study attributes. According to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria 183 , the following six bias sources were quantified: representativeness of the study population, exposure assessment, outcome ascertainment, reverse causation, control for confounding, and selection bias. Representativeness was quantified by whether the study sample came from a location that was representative of the underlying geography. Exposure assessment was quantified by whether alcohol consumption was recorded once or more than once in conventional observational studies, or with only one or multiple SNPs in MR studies. Outcome ascertainment was quantified by whether IHD was ascertained by self-report only or by at least one other measurement method. Reverse causation was quantified by whether increased IHD risk among participants who reduced or stopped drinking was accounted for (e.g., by separating former drinkers from lifetime abstainers). Control for confounding factors was quantified by which and how many covariates the effect sizes were adjusted for (i.e., through stratification, matching, weighting, or standardization). Because the most adjusted effect sizes in each study were extracted in the systematic review process and thus may have been adjusted for mediators, we additionally quantified a bias covariate for each of the following potential mediators of the alcohol-IHD relationship: body mass index, blood pressure, cholesterol (excluding high-density lipoprotein cholesterol), fibrinogen, apolipoprotein A1, and adiponectin. Selection bias was quantified by whether study participants were selected and included based on pre-existing disease states. We also quantified and considered as possible bias covariates whether the reference group was non-drinkers, including lifetime abstainers and former drinkers; whether the sample was under or over 50 years of age; whether IHD morbidity, mortality, or both endpoints were used; whether the outcome mapped to IHD or referred only to subtypes of IHD; whether the outcome mapped to MI; and what study design (cohort or case-control) was used when conventional observational studies were pooled. Details on quantified bias covariates for all included studies are provided in Supplementary Information section 5 (Tables S7 and S8 ). Using a Lasso approach 184 , the bias covariates were first ranked. They were then included sequentially, based on their ranking, as effect modifiers of the ‘signal’ obtained in step two in a linear meta-regression. Significant bias covariates were included in modeling the final risk curve. Technical details of the Lasso procedure are described elsewhere 32 .
Quantifying between-study heterogeneity, accounting for heterogeneity, uncertainty, and small number of studies
In step four, the between-study heterogeneity was quantified, accounting for heterogeneity, uncertainty, and small number of studies. In a final linear mixed-effects model, the log RRs were regressed against the ‘signal’ and selected bias covariates, with a random intercept to account for within-study correlation and a study-specific random slope with respect to the ‘signal’ to account for between-study heterogeneity. A Fisher information matrix was used to estimate the uncertainty associated with between-study heterogeneity 185 because heterogeneity is easily underestimated or may be zero when only a small number of studies are available. We estimated the mean risk curve with a 95% UI that incorporated between-study heterogeneity, and we additionally estimated a 95% UI without between-study heterogeneity as done in conventional meta-regressions (see Supplementary Information Section 7 , Table S10 ). The 95% UI incorporating between-study heterogeneity was calculated from the posterior uncertainty of the fixed effects (i.e., the ‘signal’ and selected bias covariates) and the 95% quantile of the between-study heterogeneity. The estimate of between-study heterogeneity and the estimate of the uncertainty of the between-study heterogeneity were used to determine the 95% quantile of the between-study heterogeneity. Technical details of quantifying uncertainty of between-study heterogeneity are described elsewhere 32 .
Evaluating potential for publication or reporting bias
In step five, the potential for publication or reporting bias was evaluated. The trimming algorithm used in step two helps protect against these biases, so risk curves found to have publication or reporting bias using the following methods were derived from data that still had bias even after trimming. Publication or reporting bias was evaluated using Egger’s regression 34 and visual inspection using funnel plots. Egger’s regression tested for a significant correlation between residuals of the RR estimates and their standard errors. Funnel plots showed the residuals of the risk curve against their standard errors. We reported publication or reporting bias when identified.
Estimating the burden of proof risk function
In step six, the BPRF was calculated for risk-outcome relationships that were statistically significant when evaluating the conventional 95% UI without between-study heterogeneity. The BPRF is either the 5th (if harmful) or the 95th (if protective) quantile curve inclusive of between-study heterogeneity that is closest to the RR line at 1 (i.e., null); it indicates a conservative estimate of a harmful or protective association at each exposure level, based on the available evidence. The mean risk curve, 95% UIs (with and without between-study heterogeneity), and BPRF (where applicable) are visualized along with included effect sizes using the midpoint of each alternative exposure range (trimmed data points are marked with a red x), with alcohol consumption in g/day on the x-axis and (log)RR on the y-axis.
We calculated the ROS as the average log RR of the BPRF between the 15th and 85th percentiles of alcohol exposure observed in the study data. The ROS summarizes the association of the exposure with the health outcome in a single measure. A higher, positive ROS indicates a larger association, while a negative ROS indicates a weak association. The ROS is identical for protective and harmful risks since it is based on the magnitude of the log RR. For example, a mean log BPRF between the 15th and 85th percentiles of exposure of −0.6 (protective association) and a mean log BPRF of 0.6 (harmful association) would both correspond to a ROS of 0.6. The ROS was then translated into a star rating, representing a conservative interpretation of all available evidence. A star rating of 1 (ROS: <0) indicates weak evidence of an association, a star rating of 2 (ROS: 0–0.14) indicates a >0–15% increased or >0–13% decreased risk, a star rating of 3 (ROS: >0.14–0.41) indicates a >15–50% increased or >13–34% decreased risk, a star rating of 4 (ROS: >0.41–0.62) indicates a >50–85% increased or >34–46% decreased risk, and a star rating of 5 (ROS: >0.62) indicates a >85% increased or >46% decreased risk.
Statistics & reproducibility
The statistical analyses conducted in this study are described above in detail. No statistical method was used to predetermine the sample size. When analyzing data from cohort and case-control studies, we excluded 10% of observations using a trimming algorithm; when analyzing data from MR studies, we did not exclude any observations. As all data used in this meta-analysis were from observational studies, no experiments were conducted, and no randomization or blinding took place.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The findings from this study were produced using data extracted from published literature. The relevant studies were identified through a systematic literature review and can all be accessed online as referenced in the current paper 26 , 27 , 28 , 29 , 31 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 . Further details on the relevant studies can be found on the GHDx website ( https://ghdx.healthdata.org/record/ihme-data/gbd-alcohol-ihd-bop-risk-outcome-scores ). Study characteristics of all relevant studies included in the analyses are also provided in Supplementary Information Section 4 (Tables S5 and S6 ). The template of the data collection form is provided in Supplementary Information section 3 (Table S4 ). The source data includes processed data from these studies that underlie our estimates. Source data are provided with this paper.
Code availability
Analyses were carried out using R version 4.0.5 and Python version 3.10.9. All code used for these analyses is publicly available online ( https://github.com/ihmeuw-msca/burden-of-proof ).
Bryazka, D. et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet 400 , 185–235 (2022).
Article Google Scholar
World Health Organization. Global Status Report on Alcohol and Health 2018 . (World Health Organization, Geneva, Switzerland, 2019).
Bagnardi, V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br. J. Cancer 112 , 580–593 (2015).
Article CAS PubMed Google Scholar
Wood, A. M. et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391 , 1513–1523 (2018).
Article PubMed PubMed Central Google Scholar
Goel, S., Sharma, A. & Garg, A. Effect of alcohol consumption on cardiovascular health. Curr. Cardiol. Rep. 20 , 19 (2018).
Article PubMed Google Scholar
Manthey, J. et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 393 , 2493–2502 (2019).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
Brien, S. E., Ronksley, P. E., Turner, B. J., Mukamal, K. J. & Ghali, W. A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 342 , d636 (2011).
Rehm, J. et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction 112 , 968–1001 (2017).
Roerecke, M. & Rehm, J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am. J. Epidemiol. 171 , 633–644 (2010).
Hernan, M. A. & Robin, J. M. Causal Inference: What If . (CRC Press, 2023).
Marmot, M. Alcohol and coronary heart disease. Int. J. Epidemiol. 13 , 160–167 (1984).
Shaper, A. G., Wannamethee, G. & Walker, M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 332 , 1267–1273 (1988).
Davis, C. G., Thake, J. & Vilhena, N. Social desirability biases in self-reported alcohol consumption and harms. Addict. Behav. 35 , 302–311 (2010).
Mansournia, M. A., Etminan, M., Danaei, G., Kaufman, J. S. & Collins, G. Handling time varying confounding in observational research. BMJ 359 , j4587 (2017).
Ilomäki, J. et al. Relationship between alcohol consumption and myocardial infarction among ageing men using a marginal structural model. Eur. J. Public Health 22 , 825–830 (2012).
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27 , 1133–1163 (2008).
Article MathSciNet PubMed Google Scholar
Burgess, S., Timpson, N. J., Ebrahim, S. & Davey Smith, G. Mendelian randomization: where are we now and where are we going? Int. J. Epidemiol. 44 , 379–388 (2015).
Sleiman, P. M. & Grant, S. F. Mendelian randomization in the era of genomewide association studies. Clin. Chem. 56 , 723–728 (2010).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362 , k601 (2018).
de Leeuw, C., Savage, J., Bucur, I. G., Heskes, T. & Posthuma, D. Understanding the assumptions underlying Mendelian randomization. Eur. J. Hum. Genet. 30 , 653–660 (2022).
Sheehan, N. A., Didelez, V., Burton, P. R. & Tobin, M. D. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 5 , e177 (2008).
Van de Luitgaarden, I. A. et al. Alcohol consumption in relation to cardiovascular diseases and mortality: a systematic review of Mendelian randomization studies. Eur. J. Epidemiol. 1–15 (2021).
Edenberg, H. J. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 30 , 5–13 (2007).
PubMed PubMed Central Google Scholar
Gelernter, J. et al. Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol. Psychiatry 86 , 365–376 (2019).
Article CAS PubMed PubMed Central Google Scholar
Millwood, I. Y. et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 393 , 1831–1842 (2019).
Au Yeung, S. L. et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS ONE 8 , e68054 (2013).
Article ADS PubMed PubMed Central Google Scholar
Lankester, J., Zanetti, D., Ingelsson, E. & Assimes, T. L. Alcohol use and cardiometabolic risk in the UK Biobank: a Mendelian randomization study. PLoS ONE 16 , e0255801 (2021).
Cho, Y. et al. Alcohol intake and cardiovascular risk factors: a Mendelian randomisation study. Sci. Rep. 5 , 18422 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Holmes, M. V. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 349 , g4164 (2014).
Biddinger, K. J. et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw. Open 5 , e223849–e223849 (2022).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. 28 , 2038–2044 (2022).
Zheng, P., Barber, R., Sorensen, R. J., Murray, C. J. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph. Stat. 30 , 544–556 (2021).
Article MathSciNet Google Scholar
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315 , 629–634 (1997).
Roerecke, M. & Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 12 , 182 (2014).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10 , 89 (2021).
Stevens, G. A. et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. 13 , e1002056 (2016).
Griswold, M. G. et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392 , 1015–1035 (2018).
Albert, C. M. et al. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation 100 , 944–950 (1999).
Arriola, L. et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart 96 , 124–130 (2010).
Bazzano, L. A. et al. Alcohol consumption and risk of coronary heart disease among Chinese men. Int. J. Cardiol. 135 , 78–85 (2009).
Bell, S. et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ 356 , j909 (2017).
Bergmann, M. M. et al. The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int. J. Epidemiol. 42 , 1772–1790 (2013).
Beulens, J. W. J. et al. Alcohol consumption and risk for coronary heart disease among men with hypertension. Ann. Intern. Med. 146 , 10–19 (2007).
Bobak, M. et al. Alcohol, drinking pattern and all-cause, cardiovascular and alcohol-related mortality in Eastern Europe. Eur. J. Epidemiol. 31 , 21–30 (2016).
Boffetta, P. & Garfinkel, L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology 1 , 342–348 (1990).
Britton, A. & Marmot, M. Different measures of alcohol consumption and risk of coronary heart disease and all-cause mortality: 11-year follow-up of the Whitehall II Cohort Study. Addiction 99 , 109–116 (2004).
Camargo, C. A. et al. Moderate alcohol consumption and risk for angina pectoris or myocardial infarction in U.S. male physicians. Ann. Intern. Med. 126 , 372–375 (1997).
Chang, J. Y., Choi, S. & Park, S. M. Association of change in alcohol consumption with cardiovascular disease and mortality among initial nondrinkers. Sci. Rep. 10 , 13419 (2020).
Chiuve, S. E. et al. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm 7 , 1374–1380 (2010).
Colditz, G. A. et al. Moderate alcohol and decreased cardiovascular mortality in an elderly cohort. Am. Heart J. 109 , 886–889 (1985).
Dai, J., Mukamal, K. J., Krasnow, R. E., Swan, G. E. & Reed, T. Higher usual alcohol consumption was associated with a lower 41-y mortality risk from coronary artery disease in men independent of genetic and common environmental factors: the prospective NHLBI Twin Study. Am. J. Clin. Nutr. 102 , 31–39 (2015).
Dam, M. K. et al. Five year change in alcohol intake and risk of breast cancer and coronary heart disease among postmenopausal women: prospective cohort study. BMJ 353 , i2314 (2016).
Degerud, E. et al. Associations of binge drinking with the risks of ischemic heart disease and stroke: a study of pooled Norwegian Health Surveys. Am. J. Epidemiol. 190 , 1592–1603 (2021).
de Labry, L. O. et al. Alcohol consumption and mortality in an American male population: recovering the U-shaped curve–findings from the normative Aging Study. J. Stud. Alcohol 53 , 25–32 (1992).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to alcohol consumption: a prospective study among male British doctors. Int. J. Epidemiol. 34 , 199–204 (2005).
Dyer, A. R. et al. Alcohol consumption and 17-year mortality in the Chicago Western Electric Company study. Prev. Med. 9 , 78–90 (1980).
Ebbert, J. O., Janney, C. A., Sellers, T. A., Folsom, A. R. & Cerhan, J. R. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. J. Gen. Intern. Med. 20 , 14–20 (2005).
Ebrahim, S. et al. Alcohol dehydrogenase type 1 C (ADH1C) variants, alcohol consumption traits, HDL-cholesterol and risk of coronary heart disease in women and men: British Women’s Heart and Health Study and Caerphilly cohorts. Atherosclerosis 196 , 871–878 (2008).
Friedman, L. A. & Kimball, A. W. Coronary heart disease mortality and alcohol consumption in Framingham. Am. J. Epidemiol. 124 , 481–489 (1986).
Fuchs, F. D. et al. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 160 , 466–474 (2004).
Garfinkel, L., Boffetta, P. & Stellman, S. D. Alcohol and breast cancer: a cohort study. Prev. Med. 17 , 686–693 (1988).
Gémes, K. et al. Alcohol consumption is associated with a lower incidence of acute myocardial infarction: results from a large prospective population-based study in Norway. J. Intern. Med. 279 , 365–375 (2016).
Gigleux, I. et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J. Nutr. 136 , 3027–3032 (2006).
Goldberg, R. J., Burchfiel, C. M., Reed, D. M., Wergowske, G. & Chiu, D. A prospective study of the health effects of alcohol consumption in middle-aged and elderly men. The Honolulu Heart Program. Circulation 89 , 651–659 (1994).
Goldberg, R. J. et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch. Intern. Med. 155 , 686–694 (1995).
Gordon, T. & Doyle, J. T. Drinking and coronary heart disease: the Albany Study. Am. Heart J. 110 , 331–334 (1985).
Gun, R. T., Pratt, N., Ryan, P., Gordon, I. & Roder, D. Tobacco and alcohol-related mortality in men: estimates from the Australian cohort of petroleum industry workers. Aust. N.Z. J. Public Health 30 , 318–324 (2006).
Harriss, L. R. et al. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction 102 , 1574–1585 (2007).
Hart, C. L. & Smith, G. D. Alcohol consumption and mortality and hospital admissions in men from the Midspan collaborative cohort study. Addiction 103 , 1979–1986 (2008).
Henderson, S. O. et al. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS ONE 2 , e377 (2007).
Hippe, M. et al. Familial predisposition and susceptibility to the effect of other risk factors for myocardial infarction. J. Epidemiol. Community Health 53 , 269–276 (1999).
Ikehara, S. et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: the Japan collaborative cohort study. Stroke 39 , 2936–2942 (2008).
Ikehara, S. et al. Alcohol consumption, social support, and risk of stroke and coronary heart disease among Japanese men: the JPHC Study. Alcohol. Clin. Exp. Res. 33 , 1025–1032 (2009).
Iso, H. et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke 26 , 767–773 (1995).
Jakovljević, B., Stojanov, V., Paunović, K., Belojević, G. & Milić, N. Alcohol consumption and mortality in Serbia: twenty-year follow-up study. Croat. Med. J. 45 , 764–768 (2004).
PubMed Google Scholar
Keil, U., Chambless, L. E., Döring, A., Filipiak, B. & Stieber, J. The relation of alcohol intake to coronary heart disease and all-cause mortality in a beer-drinking population. Epidemiology 8 , 150–156 (1997).
Key, T. J. et al. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 89 , 1613S–1619S (2009).
Kitamura, A. et al. Alcohol intake and premature coronary heart disease in urban Japanese men. Am. J. Epidemiol. 147 , 59–65 (1998).
Kivelä, S. L. et al. Alcohol consumption and mortality in aging or aged Finnish men. J. Clin. Epidemiol. 42 , 61–68 (1989).
Klatsky, A. L. et al. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. Am. J. Cardiol. 96 , 346–351 (2005).
Kono, S., Ikeda, M., Tokudome, S., Nishizumi, M. & Kuratsune, M. Alcohol and mortality: a cohort study of male Japanese physicians. Int. J. Epidemiol. 15 , 527–532 (1986).
Kunutsor, S. K. et al. Self-reported alcohol consumption, carbohydrate deficient transferrin and risk of cardiovascular disease: the PREVEND prospective cohort study. Clin. Chim. Acta 520 , 1–7 (2021).
Kurl, S., Jae, S. Y., Voutilainen, A. & Laukkanen, J. A. The combined effect of blood pressure and C-reactive protein with the risk of mortality from coronary heart and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 31 , 2051–2057 (2021).
Larsson, S. C., Wallin, A. & Wolk, A. Contrasting association between alcohol consumption and risk of myocardial infarction and heart failure: two prospective cohorts. Int. J. Cardiol. 231 , 207–210 (2017).
Lazarus, N. B., Kaplan, G. A., Cohen, R. D. & Leu, D. J. Change in alcohol consumption and risk of death from all causes and from ischaemic heart disease. BMJ 303 , 553–556 (1991).
Lee, D.-H., Folsom, A. R. & Jacobs, D. R. Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: the Iowa Women’s Health Study. Diabetologia 47 , 185–194 (2004).
Liao, Y., McGee, D. L., Cao, G. & Cooper, R. S. Alcohol intake and mortality: findings from the National Health Interview Surveys (1988 and 1990). Am. J. Epidemiol. 151 , 651–659 (2000).
Licaj, I. et al. Alcohol consumption over time and mortality in the Swedish Women’s Lifestyle and Health cohort. BMJ Open 6 , e012862 (2016).
Lindschou Hansen, J. et al. Alcohol intake and risk of acute coronary syndrome and mortality in men and women with and without hypertension. Eur. J. Epidemiol. 26 , 439–447 (2011).
Makelä, P., Paljärvi, T. & Poikolainen, K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: a follow-up study in a population with a low consumption level. J. Stud. Alcohol 66 , 722–728 (2005).
Malyutina, S. et al. Relation between heavy and binge drinking and all-cause and cardiovascular mortality in Novosibirsk, Russia: a prospective cohort study. Lancet 360 , 1448–1454 (2002).
Maraldi, C. et al. Impact of inflammation on the relationship among alcohol consumption, mortality, and cardiac events: the health, aging, and body composition study. Arch. Intern. Med. 166 , 1490–1497 (2006).
Marques-Vidal, P. et al. Alcohol consumption and cardiovascular disease: differential effects in France and Northern Ireland. The PRIME study. Eur. J. Cardiovasc. Prev. Rehabil. 11 , 336–343 (2004).
Meisinger, C., Döring, A., Schneider, A., Löwel, H. & KORA Study Group. Serum gamma-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis 189 , 297–302 (2006).
Merry, A. H. H. et al. Smoking, alcohol consumption, physical activity, and family history and the risks of acute myocardial infarction and unstable angina pectoris: a prospective cohort study. BMC Cardiovasc. Disord. 11 , 13 (2011).
Miller, G. J., Beckles, G. L., Maude, G. H. & Carson, D. C. Alcohol consumption: protection against coronary heart disease and risks to health. Int. J. Epidemiol. 19 , 923–930 (1990).
Mukamal, K. J., Chiuve, S. E. & Rimm, E. B. Alcohol consumption and risk for coronary heart disease in men with healthy lifestyles. Arch. Intern. Med. 166 , 2145–2150 (2006).
Ng, R., Sutradhar, R., Yao, Z., Wodchis, W. P. & Rosella, L. C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 49 , 113–130 (2020).
Onat, A. et al. Moderate and heavy alcohol consumption among Turks: long-term impact on mortality and cardiometabolic risk. Arch. Turkish Soc. Cardiol. 37 , 83–90 (2009).
Google Scholar
Pedersen, J. Ø., Heitmann, B. L., Schnohr, P. & Grønbaek, M. The combined influence of leisure-time physical activity and weekly alcohol intake on fatal ischaemic heart disease and all-cause mortality. Eur. Heart J. 29 , 204–212 (2008).
Reddiess, P. et al. Alcohol consumption and risk of cardiovascular outcomes and bleeding in patients with established atrial fibrillation. Can. Med. Assoc. J. 193 , E117–E123 (2021).
Article CAS Google Scholar
Rehm, J. T., Bondy, S. J., Sempos, C. T. & Vuong, C. V. Alcohol consumption and coronary heart disease morbidity and mortality. Am. J. Epidemiol. 146 , 495–501 (1997).
Renaud, S. C., Guéguen, R., Schenker, J. & d’Houtaud, A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology 9 , 184–188 (1998).
Ricci, C. et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ 361 , k934 (2018).
Rimm, E. B. et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 338 , 464–468 (1991).
Roerecke, M. et al. Heavy drinking occasions in relation to ischaemic heart disease mortality– an 11-22 year follow-up of the 1984 and 1995 US National Alcohol Surveys. Int. J. Epidemiol. 40 , 1401–1410 (2011).
Romelsjö, A., Allebeck, P., Andréasson, S. & Leifman, A. Alcohol, mortality and cardiovascular events in a 35 year follow-up of a nationwide representative cohort of 50,000 Swedish conscripts up to age 55. Alcohol Alcohol. 47 , 322–327 (2012).
Rostron, B. Alcohol consumption and mortality risks in the USA. Alcohol Alcohol. 47 , 334–339 (2012).
Ruidavets, J.-B. et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). BMJ 341 , c6077 (2010).
Schooling, C. M. et al. Moderate alcohol use and mortality from ischaemic heart disease: a prospective study in older Chinese people. PLoS ONE 3 , e2370 (2008).
Schutte, R., Smith, L. & Wannamethee, G. Alcohol - The myth of cardiovascular protection. Clin. Nutr. 41 , 348–355 (2022).
Sempos, C., Rehm, J., Crespo, C. & Trevisan, M. No protective effect of alcohol consumption on coronary heart disease (CHD) in African Americans: average volume of drinking over the life course and CHD morbidity and mortality in a U.S. national cohort. Contemp. Drug Probl. 29 , 805–820 (2002).
Shaper, A. G., Wannamethee, G. & Walker, M. Alcohol and coronary heart disease: a perspective from the British Regional Heart Study. Int. J. Epidemiol. 23 , 482–494 (1994).
Simons, L. A., McCallum, J., Friedlander, Y. & Simons, J. Alcohol intake and survival in the elderly: a 77 month follow-up in the Dubbo study. Aust. N.Z. J. Med. 26 , 662–670 (1996).
Skov-Ettrup, L. S., Eliasen, M., Ekholm, O., Grønbæk, M. & Tolstrup, J. S. Binge drinking, drinking frequency, and risk of ischaemic heart disease: a population-based cohort study. Scand. J. Public Health 39 , 880–887 (2011).
Snow, W. M., Murray, R., Ekuma, O., Tyas, S. L. & Barnes, G. E. Alcohol use and cardiovascular health outcomes: a comparison across age and gender in the Winnipeg Health and Drinking Survey Cohort. Age Ageing 38 , 206–212 (2009).
Song, R. J. et al. Alcohol consumption and risk of coronary artery disease (from the Million Veteran Program). Am. J. Cardiol. 121 , 1162–1168 (2018).
Streppel, M. T., Ocké, M. C., Boshuizen, H. C., Kok, F. J. & Kromhout, D. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: the Zutphen Study. J. Epidemiol. Community Health 63 , 534–540 (2009).
Suhonen, O., Aromaa, A., Reunanen, A. & Knekt, P. Alcohol consumption and sudden coronary death in middle-aged Finnish men. Acta Med. Scand. 221 , 335–341 (1987).
Thun, M. J. et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N. Engl. J. Med. 337 , 1705–1714 (1997).
Tolstrup, J. et al. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ 332 , 1244–1248 (2006).
Wannamethee, G. & Shaper, A. G. Alcohol and sudden cardiac death. Br. Heart J. 68 , 443–448 (1992).
Wannamethee, S. G. & Shaper, A. G. Type of alcoholic drink and risk of major coronary heart disease events and all-cause mortality. Am. J. Public Health 89 , 685–690 (1999).
Wilkins, K. Moderate alcohol consumption and heart disease. Health Rep. 14 , 9–24 (2002).
Yang, L. et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int. J. Epidemiol. 41 , 1101–1113 (2012).
Yi, S. W., Yoo, S. H., Sull, J. W. & Ohrr, H. Association between alcohol drinking and cardiovascular disease mortality and all-cause mortality: Kangwha Cohort Study. J. Prev. Med. Public Health 37 , 120–126 (2004).
Younis, J., Cooper, J. A., Miller, G. J., Humphries, S. E. & Talmud, P. J. Genetic variation in alcohol dehydrogenase 1C and the beneficial effect of alcohol intake on coronary heart disease risk in the Second Northwick Park Heart Study. Atherosclerosis 180 , 225–232 (2005).
Yusuf, S. et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395 , 795–808 (2020).
Zhang, Y. et al. Association of drinking pattern with risk of coronary heart disease incidence in the middle-aged and older Chinese men: results from the Dongfeng-Tongji cohort. PLoS ONE 12 , e0178070 (2017).
Augustin, L. S. A. et al. Alcohol consumption and acute myocardial infarction: a benefit of alcohol consumed with meals? Epidemiology 15 , 767–769 (2004).
Bianchi, C., Negri, E., La Vecchia, C. & Franceschi, S. Alcohol consumption and the risk of acute myocardial infarction in women. J. Epidemiol. Community Health 47 , 308–311 (1993).
Brenner, H. et al. Coronary heart disease risk reduction in a predominantly beer-drinking population. Epidemiology 12 , 390–395 (2001).
Dorn, J. M. et al. Alcohol drinking pattern and non-fatal myocardial infarction in women. Addiction 102 , 730–739 (2007).
Fan, A. Z., Ruan, W. J. & Chou, S. P. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev. Med. 118 , 336–343 (2019).
Fumeron, F. et al. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J. Clin. Investig. 96 , 1664–1671 (1995).
Gaziano, J. M. et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N. Engl. J. Med. 329 , 1829–1834 (1993).
Genchev, G. D., Georgieva, L. M., Weijenberg, M. P. & Powles, J. W. Does alcohol protect against ischaemic heart disease in Bulgaria? A case-control study of non-fatal myocardial infarction in Sofia. Cent. Eur. J. Public Health 9 , 83–86 (2001).
CAS PubMed Google Scholar
Hammar, N., Romelsjö, A. & Alfredsson, L. Alcohol consumption, drinking pattern and acute myocardial infarction. A case referent study based on the Swedish Twin Register. J. Intern. Med. 241 , 125–131 (1997).
Hines, L. M. et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N. Engl. J. Med. 344 , 549–555 (2001).
Ilic, M., Grujicic Sipetic, S., Ristic, B. & Ilic, I. Myocardial infarction and alcohol consumption: a case-control study. PLoS ONE 13 , e0198129 (2018).
Jackson, R., Scragg, R. & Beaglehole, R. Alcohol consumption and risk of coronary heart disease. BMJ 303 , 211–216 (1991).
Kabagambe, E. K., Baylin, A., Ruiz-Narvaez, E., Rimm, E. B. & Campos, H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am. J. Clin. Nutr. 82 , 1336–1345 (2005).
Kalandidi, A. et al. A case-control study of coronary heart disease in Athens, Greece. Int. J. Epidemiol. 21 , 1074–1080 (1992).
Kaufman, D. W., Rosenberg, L., Helmrich, S. P. & Shapiro, S. Alcoholic beverages and myocardial infarction in young men. Am. J. Epidemiol. 121 , 548–554 (1985).
Kawanishi, M., Nakamoto, A., Konemori, G., Horiuchi, I. & Kajiyama, G. Coronary sclerosis risk factors in males with special reference to lipoproteins and apoproteins: establishing an index. Hiroshima J. Med. Sci. 39 , 61–64 (1990).
Kono, S. et al. Alcohol intake and nonfatal acute myocardial infarction in Japan. Am. J. Cardiol. 68 , 1011–1014 (1991).
Mehlig, K. et al. CETP TaqIB genotype modifies the association between alcohol and coronary heart disease: the INTERGENE case-control study. Alcohol 48 , 695–700 (2014).
Miyake, Y. Risk factors for non-fatal acute myocardial infarction in middle-aged and older Japanese. Fukuoka Heart Study Group. Jpn. Circ. J. 64 , 103–109 (2000).
Oliveira, A., Barros, H., Azevedo, A., Bastos, J. & Lopes, C. Impact of risk factors for non-fatal acute myocardial infarction. Eur. J. Epidemiol. 24 , 425–432 (2009).
Oliveira, A., Barros, H. & Lopes, C. Gender heterogeneity in the association between lifestyles and non-fatal acute myocardial infarction. Public Health Nutr. 12 , 1799–1806 (2009).
Romelsjö, A. et al. Abstention, alcohol use and risk of myocardial infarction in men and women taking account of social support and working conditions: the SHEEP case-control study. Addiction 98 , 1453–1462 (2003).
Schröder, H. et al. Myocardial infarction and alcohol consumption: a population-based case-control study. Nutr. Metab. Cardiovasc. Dis. 17 , 609–615 (2007).
Scragg, R., Stewart, A., Jackson, R. & Beaglehole, R. Alcohol and exercise in myocardial infarction and sudden coronary death in men and women. Am. J. Epidemiol. 126 , 77–85 (1987).
Tavani, A., Bertuzzi, M., Gallus, S., Negri, E. & La Vecchia, C. Risk factors for non-fatal acute myocardial infarction in Italian women. Prev. Med. 39 , 128–134 (2004).
Tavani, A. et al. Intake of specific flavonoids and risk of acute myocardial infarction in Italy. Public Health Nutr. 9 , 369–374 (2006).
Zhou, X., Li, C., Xu, W., Hong, X. & Chen, J. Relation of alcohol consumption to angiographically proved coronary artery disease in chinese men. Am. J. Cardiol. 106 , 1101–1103 (2010).
Yang, Y. et al. Alcohol consumption and risk of coronary artery disease: a dose-response meta-analysis of prospective studies. Nutrition 32 , 637–644 (2016).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4 , 330–345 (2017).
Mukamal, K. J., Stampfer, M. J. & Rimm, E. B. Genetic instrumental variable analysis: time to call Mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur. J. Epidemiol. 35 , 93–97 (2020).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 , 693–698 (2018).
Shi, J. et al. Mendelian randomization with repeated measures of a time-varying exposure: an application of structural mean models. Epidemiology 33 , 84–94 (2022).
Burgess, S., Swanson, S. A. & Labrecque, J. A. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur. J. Epidemiol. 36 , 253–257 (2021).
Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur. J. Epidemiol. 35 , 99–111 (2020).
Burgess, S. Violation of the constant genetic effect assumption can result in biased estimates for non-linear mendelian randomization. Hum. Hered. 88 , 79–90 (2023).
Tian, H., Mason, A. M., Liu, C. & Burgess, S. Relaxing parametric assumptions for non-linear Mendelian randomization using a doubly-ranked stratification method. PLoS Genet. 19 , e1010823 (2023).
Levin, M. G. & Burgess, S. Mendelian randomization as a tool for cardiovascular research: a review. JAMA Cardiol. 9 , 79–89 (2024).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 4 , 186 (2019).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 , 304–314 (2016).
Holmes, M. V., Ala-Korpela, M. & Smith, G. D. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat. Rev. Cardiol. 14 , 577–590 (2017).
Labrecque, J. A. & Swanson, S. A. Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. Am. J. Epidemiol. 188 , 231–238 (2019).
Spiegelman, D. et al. The Moderate Alcohol and Cardiovascular Health Trial (MACH15): design and methods for a randomized trial of moderate alcohol consumption and cardiometabolic risk. Eur. J. Prev. Cardiol. 27 , 1967–1982 (2020).
DeJong, W. The Moderate Alcohol and Cardiovascular Health Trial: public health advocates should support good science, not undermine it. Eur. J. Prev. Cardiol. 28 , e22–e24 (2021).
National Institutes of Health. NIH to End Funding for Moderate Alcohol and Cardiovascular Health Trial https://www.nih.gov/news-events/news-releases/nih-end-funding-moderate-alcohol-cardiovascular-health-trial (2018).
Miller, L. M., Anderson, C. A. M. & Ix, J. H. Editorial: from MACH15 to MACH0 – a missed opportunity to understand the health effects of moderate alcohol intake. Eur. J. Prev. Cardiol. 28 , e23–e24 (2021).
Anderson, B. O. et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 8 , e6–e7 (2023).
Au Yeung, S. L. & Lam, T. H. Unite for a framework convention for alcohol control. Lancet 393 , 1778–1779 (2019).
Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 183 , 758–764 (2016).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 , e1001779 (2015).
The ARIC Investigators. The Atherosclerosis Risk in Communit (ARIC) study: design and objectives. Am. J. Epidemiol. 129 , 687–702 (1989).
Mahmood, S. S., Levy, D., Vasan, R. S. & Wang, T. J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 383 , 999–1008 (2014).
Gmel, G. & Rehm, J. Measuring alcohol consumption. Contemp. Drug Probl. 31 , 467–540 (2004).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 , 924–926 (2008).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 58 , 267–288 (1996).
Biggerstaff, B. J. & Tweedie, R. L. Incorporating variability in estimates of heterogeneity in the random effects model in meta‐analysis. Stat. Med. 16 , 753–768 (1997).
Download references
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation [OPP1152504]. S.L. has received grants or contracts from the UK Medical Research Council [MR/T017708/1], CDC Foundation [project number 996], World Health Organization [APW No 2021/1194512], and is affiliated with the NIHR Oxford Biomedical Research Centre. The University of Oxford’s Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) is supported by core grants from the Medical Research Council [Clinical Trial Service Unit A310] and the British Heart Foundation [CH/1996001/9454]. The CTSU receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the final report, or the decision to publish.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Sinclair Carr, Dana Bryazka, Susan A. McLaughlin, Peng Zheng, Aleksandr Y. Aravkin, Simon I. Hay, Hilary R. Lawlor, Erin C. Mullany, Christopher J. L. Murray, Sneha I. Nicholson, Gregory A. Roth, Reed J. D. Sorensen & Emmanuela Gakidou
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Peng Zheng, Aleksandr Y. Aravkin, Simon I. Hay, Christopher J. L. Murray, Gregory A. Roth & Emmanuela Gakidou
Clinical Trial Service Unit & Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, Oxfordshire, UK
Sarasvati Bahadursingh & Sarah Lewington
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin
Institute for Mental Health Policy Research, Centre for Addiction and Mental Health, Toronto, ON, Canada
Jürgen Rehm
Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada
Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Faculty of Medicine, Institute of Medical Science (IMS), University of Toronto, Toronto, ON, Canada
World Health Organization / Pan American Health Organization Collaborating Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada
Center for Interdisciplinary Addiction Research (ZIS), Department of Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany
Institute of Clinical Psychology and Psychotherapy, Technische Universität Dresden, Dresden, Germany
Division of Cardiology, Department of Medicine, University of Washington, Seattle, WA, USA
Gregory A. Roth
You can also search for this author in PubMed Google Scholar
Contributions
S.C., S.A.M., S.I.H., and E.C.M. managed the estimation or publications process. S.C. wrote the first draft of the manuscript. S.C. had primary responsibility for applying analytical methods to produce estimates. S.C. and H.R.L. had primary responsibility for seeking, cataloging, extracting, or cleaning data; designing or coding figures and tables. S.C., D.B., S.B., E.C.M., S.I.N., J.R., and R.J.D.S. provided data or critical feedback on data sources. S.C., D.B., P.Z., A.Y.A., S.I.N., and R.J.D.S. developed methods or computational machinery. S.C., D.B., P.Z., S.B., S.I.H., E.C.M., C.J.L.M., S.I.N., J.R., R.J.D.S., S.L., and E.G. provided critical feedback on methods or results. S.C., D.B., S.A.M., S.B., S.I.H., C.J.L.M., J.R., G.A.R., S.L., and E.G. drafted the work or revised it critically for important intellectual content. S.C., S.I.H., E.C.M., and E.G. managed the overall research enterprise.
Corresponding author
Correspondence to Sinclair Carr .
Ethics declarations
Competing interests.
G.A.R. has received support for this manuscript from the Bill and Melinda Gates Foundation [OPP1152504]. S.L. has received grants or contracts from the UK Medical Research Council [MR/T017708/1], CDC Foundation [project number 996], World Health Organization [APW No 2021/1194512], and is affiliated with the NIHR Oxford Biomedical Research Centre. The University of Oxford’s Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) is supported by core grants from the Medical Research Council [Clinical Trial Service Unit A310] and the British Heart Foundation [CH/1996001/9454]. The CTSU receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Shiu Lun Au Yeung, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Carr, S., Bryazka, D., McLaughlin, S.A. et al. A burden of proof study on alcohol consumption and ischemic heart disease. Nat Commun 15 , 4082 (2024). https://doi.org/10.1038/s41467-024-47632-7
Download citation
Received : 14 June 2023
Accepted : 08 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41467-024-47632-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
COMMENTS
Self-reported alcohol consumption is underreported in most epidemiological studies 133,134 and even the classification of drinkers as lifetime abstainers can be unreliable, with several studies in developed countries finding that the majority of self-reported lifetime abstainers are in fact former drinkers. 135,136 If this is the case, the ...
It is the alcohol that causes harm, not the beverage. Alcohol is a toxic, psychoactive, and dependence-producing substance and has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer decades ago – this is the highest risk group, which also includes asbestos, radiation and tobacco.
Alcohol use is a leading global cause of disease burden and substantial health loss. 1 Risk of all-cause mortality is positively associated with the level of alcohol consumption, such that any level of consumption is potentially harmful. 1 These findings are consistent with the well-demonstrated relationship of heavy drinking and AUD to ...
Moreover, alcohol intake in the majority of epidemiological studies is measured only once and through food frequency questionnaires or from quantity–frequency measures which may underestimate alcohol consumption, because the validity of self-reported alcohol intake has been questioned due to the fact of fear of stigmatization . These ...
In general, excessive alcohol consumption accounts for 5.1% of global diseases and injuries . According to an investigation based on gender, harmful drinking accounts for 7.1% and 2.2% of global diseases for men and women, respectively . Furthermore, alcohol consumption is also responsible for 10% of all deaths among people aged 15–49 .
Intervention studies have generally been conducted as short-term randomized controlled trials that investigated the BP effects of reduced alcohol intake in adults with a high alcohol intake. 5,7–9 The body of evidence detailing the effects of changes in alcohol intake on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in persons who were initially consuming light to moderate ...
Background Previous studies have shown inconsistent findings regarding the association of light to moderate alcohol consumption with cause-specific mortality. Therefore, this study sought to examine the prospective association of alcohol consumption with all-cause and cause-specific mortality in the US population. Methods This was a population-based cohort study of adults aged 18 years or ...
As shown in Table 1, the alcohol consumption rate is quite different between males and females.The rate of modest drinking and regular drinking in males is 22.6% and 12.1%, whereas 5.8% and 2.3 in ...
We also estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD using only data from cohort studies. In total, 95 cohort studies – of which one was a ...
In current drinkers of alcohol in high-income countries, the threshold for lowest risk of all-cause mortality was about 100 g/week. For cardiovascular disease subtypes other than myocardial infarction, there were no clear risk thresholds below which lower alcohol consumption stopped being associated with lower disease risk. These data support limits for alcohol consumption that are lower than ...